Lanthanide metal europium ion complex probe-based method for detecting pH value
A technology of lanthanide metal and detection method, which is applied in the field of chemical analysis to achieve the effects of rapid pH value response, low price and guaranteed repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
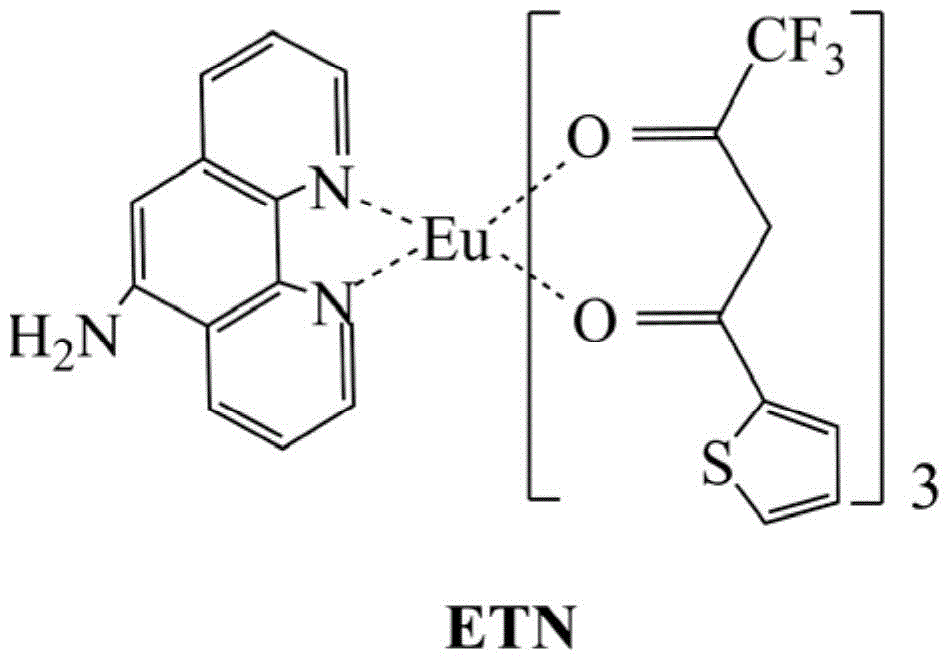
[0045] Eu(TTA) 3 (5-NH 2 -phen) preparation, concrete steps are as follows:
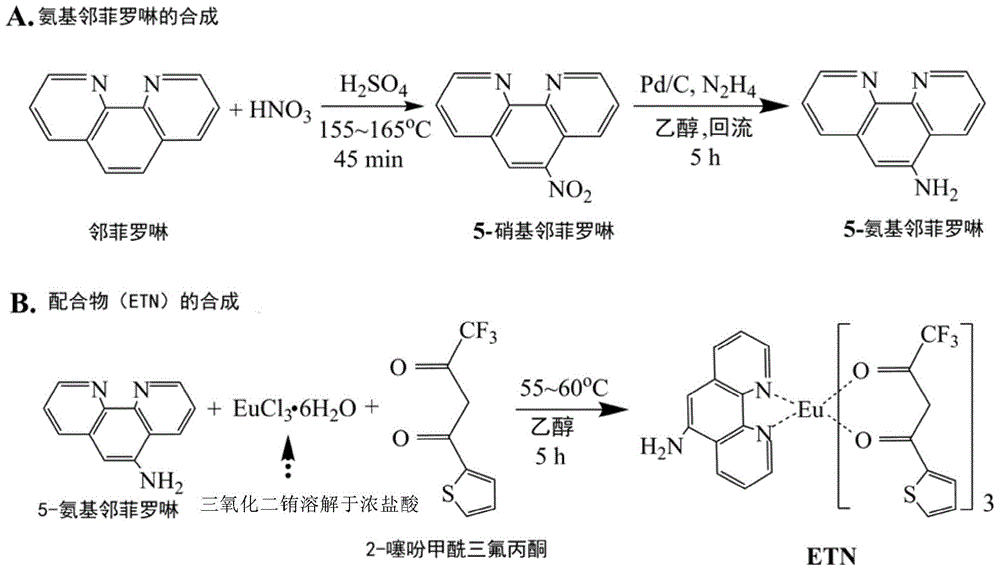
[0046] a. 5-NH with 1,10-phenanthroline (phen) as raw material 2 Synthesis of -phen
[0047] Synthetic steps: (1) Nitrolation of phen. Add 13mL of concentrated sulfuric acid to a 250mL three-necked flask, add 2.0g of phen in batches under magnetic stirring conditions, dissolve with magnetic stirring, and slowly raise the temperature. When the temperature rises to 150°C, slowly add 5.5mL of concentrated nitric acid. ℃, continue to react for 45min. After the reaction is complete, carefully pour the reaction solution into 130 g of ice-water mixture, and after the crushed ice is completely melted, use 2.0 mol·L -1 Adjust the pH value of NaOH solution to 7.0. At this time, a large amount of yellow precipitates are formed, which are filtered under reduced pressure, washed with water, and air-dried to obtain crude NO 2 -phen, recrystallized twice with ethanol to obtain yellow crystals, dried in vacuum...
Embodiment 2
[0050] Example 2: Stability study of ETN time-resolved fluorescence intensity
[0051] Take 10 different 10mL colorimetric tubes, add 1.0mL ETN stock solution respectively, and put them in a 60°C oven to remove the ethanol solvent. Then use B–R buffer with a pH value of 7.5 to adjust the volume to the mark, fully dissolve and mix evenly, and let it stand at room temperature for 10 minutes. After the ETN is fully dissolved and stabilized, take 3.0mL of the solution and add it to a 1-cm cuvette. , using a Perkin-Elmer LS-55 fluorescent phosphorescence spectrophotometer to measure and record the time-resolved fluorescence intensity changes. The maximum excitation wavelength and maximum emission wavelength are 355nm and 611nm, respectively, the slit is 5.0nm, and the delay time is 0.1ms. Thereby, 10 different time-resolved fluorescence intensity values were obtained, and the relative standard deviation was calculated, and the relative standard deviation was 3.2%, which was less ...
Embodiment 3
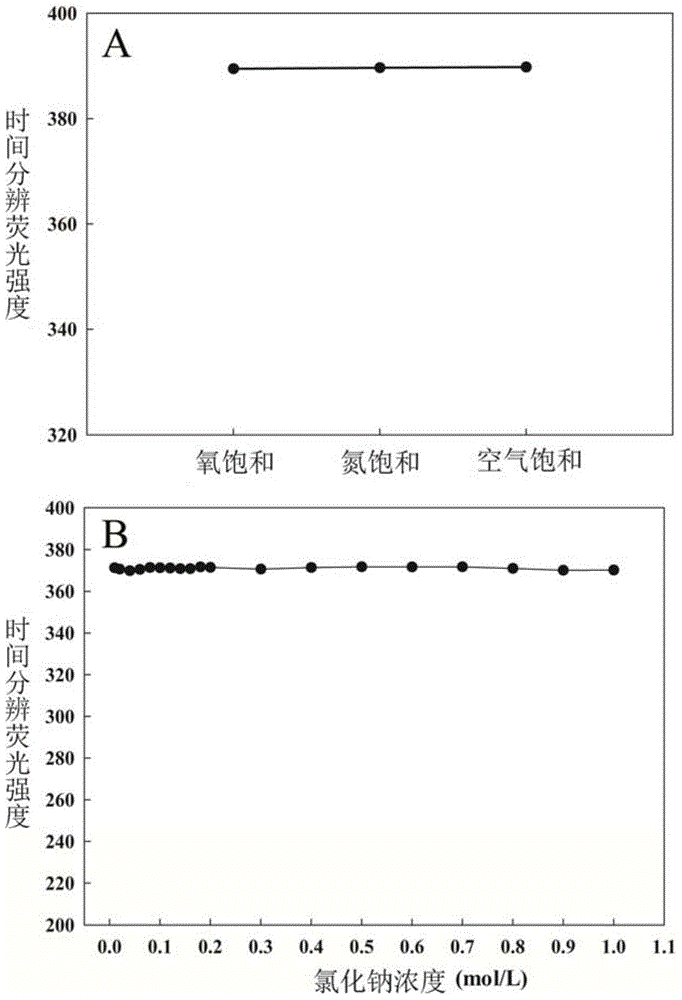
[0052] Example 3: Research on the Effect of Dissolved Oxygen and Ionic Strength
[0053] a. Effect of dissolved oxygen on the time-resolved fluorescence intensity of ETN
[0054] In this example, three different dissolved oxygen solutions were prepared, namely oxygen saturated solution, nitrogen saturated solution and air saturated solution. The pH values of the three solutions were controlled at 7.0. Then, the time-resolved fluorescence intensity of ETN in these three solutions was measured according to the steps and methods in Example 2, so as to study its influence. The results are recorded in the attached image 3 in Figure A. The results show that the time-resolved fluorescence intensities of ETN in the three solutions are basically unchanged, thereby fully illustrating that the interference of the oxygen concentration in the solution to the detection method can be ignored, and the influence of dissolved oxygen on the present invention can be ignored in actual detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com