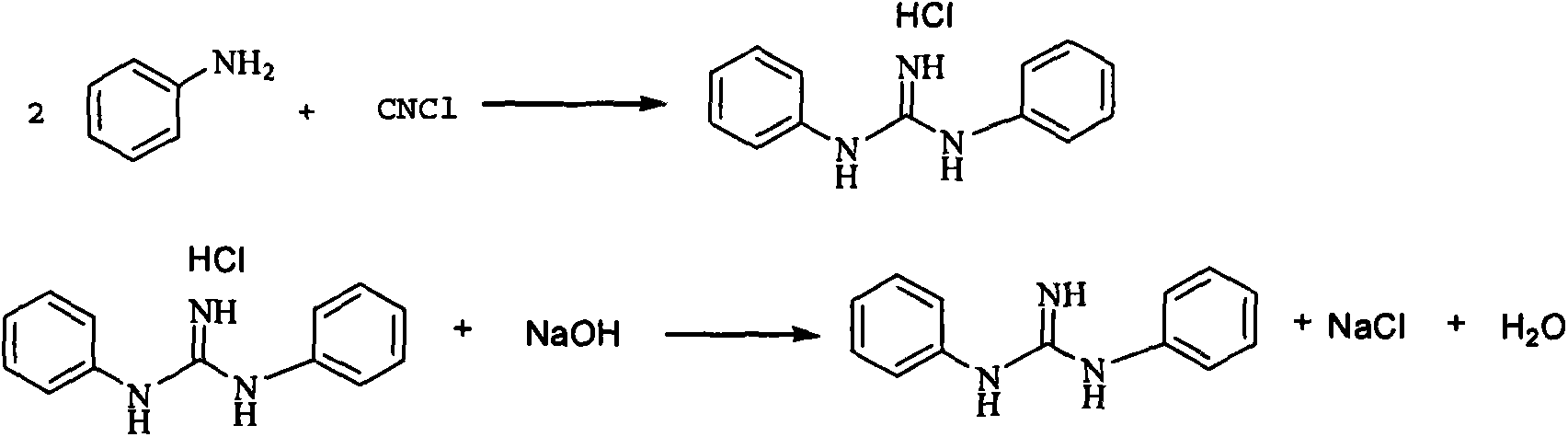
Process route for continuously synthesizing diphenylguanidine
A technology of diphenylguanidine and aniline, applied in the field of organic synthesis, to achieve the effects of high yield, good purity and reduced reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0033] Feed aniline and water into the tubular reactor at a speed of 20 g / min. The purified cyanogen chloride is contacted and reacted in the tubular reactor, the residence time of aniline in the tubular reactor is 2 minutes, and the temperature of the tubular reactor is 170°C. The crude product flows out from the tubular filter and is then filtered, neutralized with 10% sodium hydroxide to pH ≈ 7.0, washed and dried to obtain a diphenylguanidine product. The yield of diphenylguanidine is 96.5%, and the content is 99.71%.
example 2
[0035] Aniline and ethanol are fed into the tubular reactor at a rate of 20 g / min. In the tubular reactor, the purified cyanogen chloride is contacted and reacted. The residence time of aniline in the tubular reactor is 2 minutes, and the temperature of the tubular reactor is 160°C. The crude product flows out from the tubular filter and is then filtered, neutralized with 10% sodium hydroxide to pH ≈ 7.0, washed and dried to obtain a diphenylguanidine product. The yield of diphenylguanidine is 97.2%, and the content is 99.42%.
example 3
[0037] Aniline and toluene were fed into the tubular reactor at a rate of 20 g / min. In the tubular reactor, the purified cyanogen chloride is contacted and reacted. The residence time of aniline in the tubular reactor is 2.5 minutes, and the temperature of the tubular reactor is 170°C. The crude product flows out from the tubular filter and is then filtered, neutralized with 10% sodium hydroxide to pH ≈ 7.0, washed and dried to obtain a diphenylguanidine product. The yield of diphenylguanidine is 97.5%, and the content is 99.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com