Low-endotoxin escherichia coli prokaryotic expression engineering bacterial mutant strain and construction method
A technology of Escherichia coli and prokaryotic expression, applied in the field of bioengineering, can solve the problems of restricting the application of recombinant proteins, reducing the endotoxin activity of lipopolysaccharide, etc., and achieve the effects of reducing pollution, reducing toxicity, and broad market application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 1,? wxya Primer design and PCR amplification
[0064] According to the reported Escherichia coli BL21 (DE3) sequence (refer to GenBank sequence number NC_012892), design two pairs of fusion PCR primers to amplify separately wxya The upstream homology arm and downstream homology arm of the gene have amplified fragment sizes of 450bp and 380bp respectively. The above primers were synthesized by Beijing Huada Gene Company. The primer sequences are as follows:
[0065] msbB left-up: 5'CAGTTCGACAATGTGGAAGAAG 3'
[0066] msbB left-down: 5’ ACCTGCAGGATGCGGCCGCGG GCCTCTCGCGAGG3’
[0067] msbB right-up: 5’ CCGCGGCCGCATCCTGCAGGT GCTTTTCCAGTTTCGG3’
[0068] msbB right-down: 5’ GCGTTATATGCACTTGCGC 3’
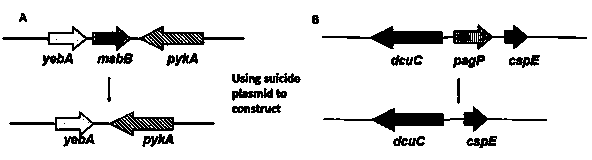
[0069] 2. Escherichia coli BL21 (DE3) wxya Amplification and fusion of the upper and lower homology arms of the gene
[0070] Inoculate the lyophilized Escherichia coli BL21 (DE3) strain into LB (ordinary broth) plate for overnight culture, pick a single colony and inocu...
Embodiment 2
[0081] 1,? pagP Primer design and PCR amplification
[0082] According to the reported Escherichia coli BL21 (DE3) sequence (refer to GenBank sequence number NC_012892), design two pairs of fusion PCR primers to amplify separately pagP The upstream homology arm and downstream homology arm of the gene have amplified fragment sizes of 350bp and 280bp respectively. The above primers were synthesized by Beijing Huada Gene Company. The primer sequences are as follows:
[0083] pagP left-up: 5’ CCTTGATTGCATTTTGTCAT 3’
[0084] pagP left-down: 5’ GTCTCACCCGGGCCTGCAGGTTGTGACCATAAAACATTTA 3’
[0085] pagP right-up: 5’ CACAACCTGCAGGCCCGGGTGAGACAAATGAAGTTTTAGT 3’
[0086] pagP right-down: 5’ TGCTGCCGTCTTCCGGAGTA 3’
[0087] 2. Escherichia coli BL21 (DE3) pagP Amplification and fusion of the upper and lower homology arms of the gene
[0088] Inoculate the lyophilized Escherichia coli BL21 (DE3) strain into LB (ordinary broth) plate for overnight culture, pick a single colony ...
Embodiment 3
[0099] 1. Construction of low-copy expression plasmids
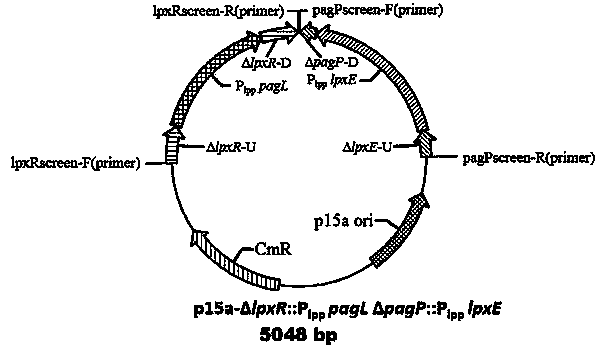
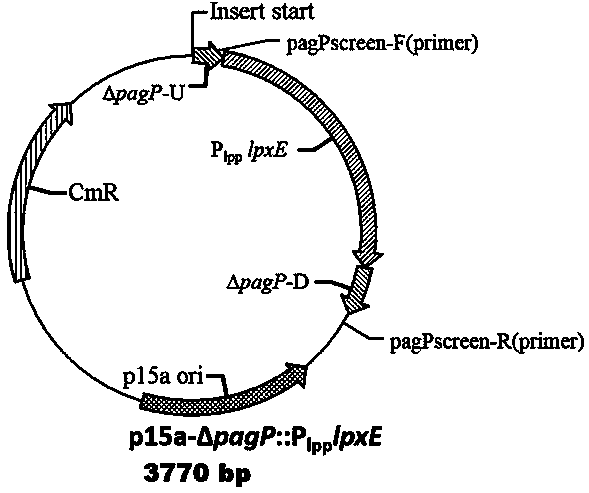
[0100] Using the plasmid pYA4291 as a template, design primers to amplify the gene containing pagL and promoter P lpp A whole sequence of lpxRU-P lpp pag L -lpxRD, use the sequence to perform +A treatment, use the plus A reaction kit of Tiangen Biochemical, the reaction system is: template 15 μL, plus A buffer 4 μL, Taq enzyme 1 μL; the reaction conditions are: 72 ° C for 30 minutes, after the reaction is completed stand-by. The fragment needs to be connected to the T-terminal expression vector p15a, and at the same time use Ahd I The vector is digested with enzymes to make it have a T-terminus, and the two fragments are connected to obtain expression pag L low-copy plasmids.
[0101] Using the same strategy, using the plasmid pYA4295 as a template, amplified containing lpxE and promoter P lpp A whole segment of the sequence was ligated into p15a in the same way to obtain a low-copy plasmid expressing lpxE.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com