Method for determining rhenium in smelted waste acid by employing complexing separation of tartaric acid hydrogen peroxide
A hydrogen peroxide and complex separation technology, applied in the field of chemical analysis, can solve the problems of low rhenium content, matrix effect and coexisting ion interference, difficulty in direct and accurate determination of smelting foul acid rhenium, etc., so as to improve the recovery rate and improve the accuracy. and reliability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
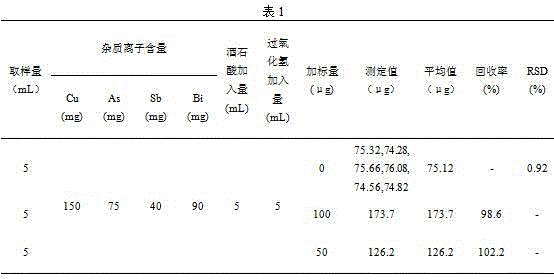
[0020] Step 1. Accurately pipette 5mL of a smelting acid solution into a 100mL beaker, add 5mL tartaric acid solution (mass volume concentration: 500g / L), shake well, and adjust the solution with sodium hydroxide solution (mass volume concentration: 600g / L) pH to weakly alkaline (pH=8-9), shake well, slowly add 5mL hydrogen peroxide solution (commercially available, 30% by volume), shake well, heat on an electric heating plate until excess peroxide Hydrogen decomposition is complete (no small bubbles are generated), add 10mL sodium hydroxide solution (mass volume concentration: 600g / L) and shake to dissolve the salts. After cooling, transfer the solution to a 60mL separatory funnel and flush to 30mL.
[0021] Step 2. Add 10 mL of acetone for extraction and shake for 3 minutes. After the solution is separated, keep the organic phase, then extract the water phase with 10 mL of acetone and shake for 3 minutes. After the solution is separated, combine the organic phase and discard ...
Embodiment 2
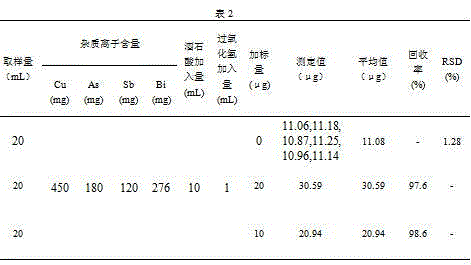
[0025] Step 1. Accurately pipette 20mL of a copper smelting dirty acid solution into a 100mL beaker, add 10mL tartaric acid solution (500g / L), shake well, and adjust the pH of the solution with sodium hydroxide solution (mass volume concentration: 600g / L) to Weak and weak alkaline (pH=8-9), shake well, slowly add 1mL hydrogen peroxide solution (commercially available), shake well, heat on the electric heating plate until the excess hydrogen peroxide is completely decomposed (no small bubbles are generated), Add 10mL sodium hydroxide solution (mass volume concentration: 600g / L) and shake to dissolve the salts. After cooling, transfer the solution to a 60mL separatory funnel and flush to 30mL.
[0026] Step 2. Add 10mL of acetone for extraction, shake for 2min, keep the organic phase after the solution is separated, then extract the water phase with 10mL of acetone, shake for 2min, after the solution is separated, combine the organic phase, discard the water phase; use 20mL Sodi...
Embodiment 3
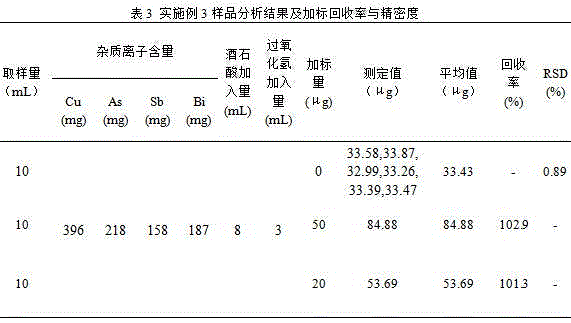
[0030] Step 1. Accurately pipette 10mL of a certain smelting acid solution into a 100mL beaker, add 8mL of tartaric acid solution (500g / L), shake well, and adjust the pH of the solution to weak with sodium hydroxide solution (mass volume concentration: 600g / L) Alkaline (PH=8-9), shake well, slowly add 3mL hydrogen peroxide solution (commercially available, 30% by volume), shake well, heat on the electric heating plate until the excess hydrogen peroxide is completely decomposed ( No small bubbles), add 10mL sodium hydroxide solution (mass volume concentration: 600g / L) and shake to dissolve the salts, transfer the solution to a 60mL separatory funnel after cooling, and flush to 30mL.
[0031] Step 2. Add 10 mL of acetone for extraction and shake for 3 minutes. After the solution is separated, keep the organic phase, then extract the water phase with 10 mL of acetone and shake for 3 minutes. After the solution is separated, combine the organic phase and discard the water phase; us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com