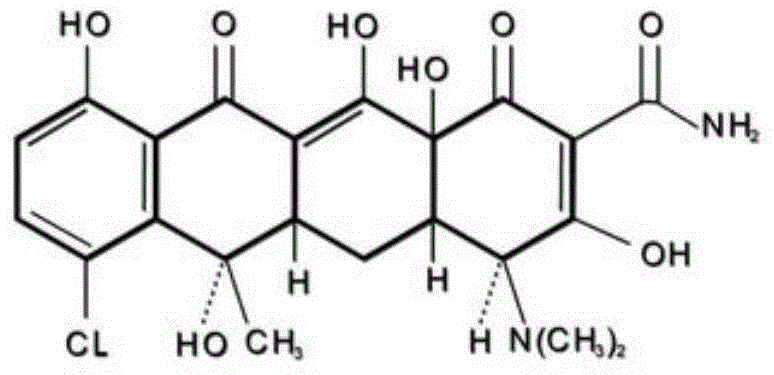
Clathrate compound of aureomycin zinc complex, and preparation method thereof
A technology of chlortetracycline zinc and complexes, which is applied in the field of drug preparation, can solve the problems of poor solubility and low dissolution rate of chlortetracycline zinc complexes, and achieve improved drug bioavailability, reduced dosage, and reduced drug side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Preparation of clathrate of the present invention
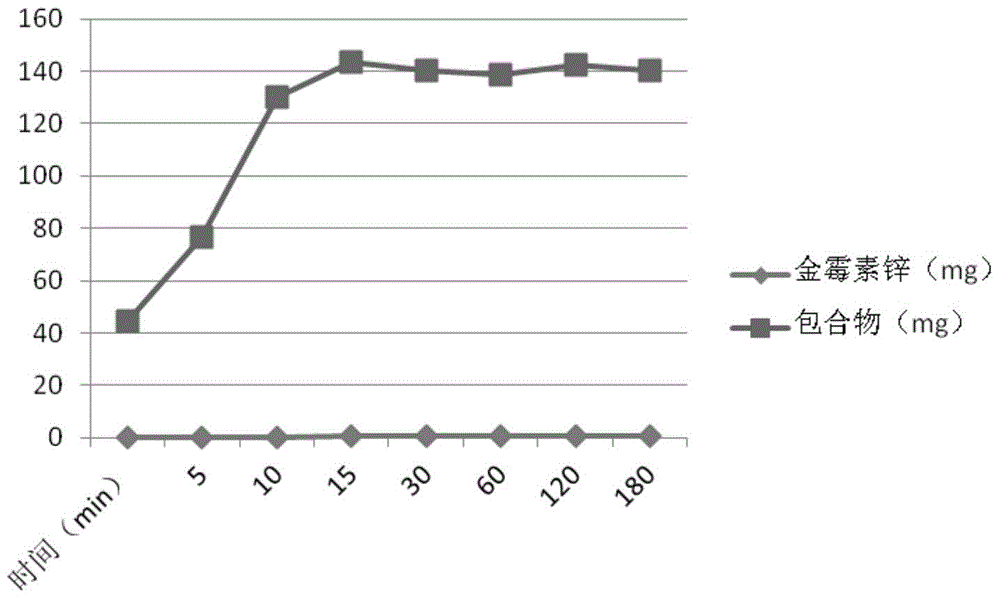
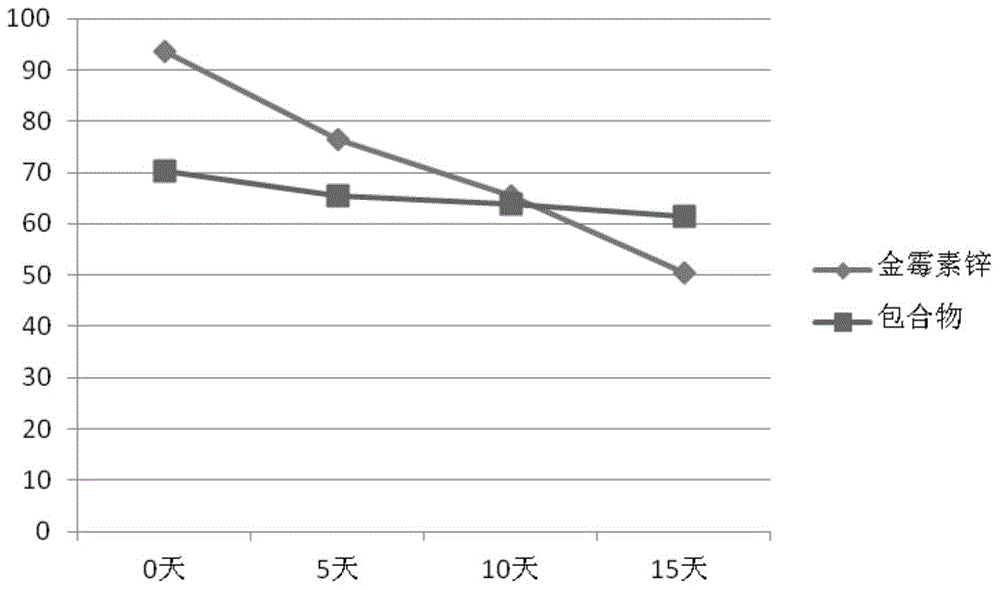
[0025] Weigh 10 grams of chlortetracycline zinc complex, add 48 ml of methanol, 48 ml of dimethyl sulfoxide, 2 ml of N,N-dimethylformamide, and 2 ml of tetrahydrofuran into a mixed organic solvent, and dissolve it completely , kept at 55°C, dripped a saturated aqueous solution containing 14 grams of hydroxypropyl-β-cyclodextrin, stirred at 55°C for 2 hours, stopped heating, and continued to stir to room temperature to obtain a yellow solution, which was filtered and spray-dried to obtain gold Inclusion complexes of mycin zinc complexes.
Embodiment 2
[0026] Embodiment 2 Preparation of clathrate of the present invention
[0027] Weigh 10 grams of chlortetracycline zinc complex, add 48 ml of methanol, 48 ml of dimethyl sulfoxide, 2 ml of N,N-dimethylformamide, and 2 ml of tetrahydrofuran into a mixed organic solvent, and dissolve it completely , kept at 60°C, dripped a saturated aqueous solution containing 28 grams of hydroxypropyl-β-cyclodextrin, continued to stir for 30 minutes, stopped heating, and continued to stir to room temperature to obtain a yellow solution, which was filtered and freeze-dried, which was Golden Mold Inclusion complexes of zinc complexes.
Embodiment 3
[0028] Embodiment 3 Preparation of clathrate of the present invention
[0029] Weigh 10 grams of chlortetracycline zinc complex, add 48 ml of methanol, 48 ml of dimethyl sulfoxide, 2 ml of N,N-dimethylformamide, and 2 ml of tetrahydrofuran into a mixed organic solvent, and dissolve it completely , kept at 60°C, dripped a saturated aqueous solution containing 28 grams of hydroxypropyl-β-cyclodextrin, ultrasonicated at 65°C for 30 minutes, filtered through a 0.45 μm membrane, and the resulting solution was spray-dried to obtain aureomycin Inclusion complexes of zinc complexes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com