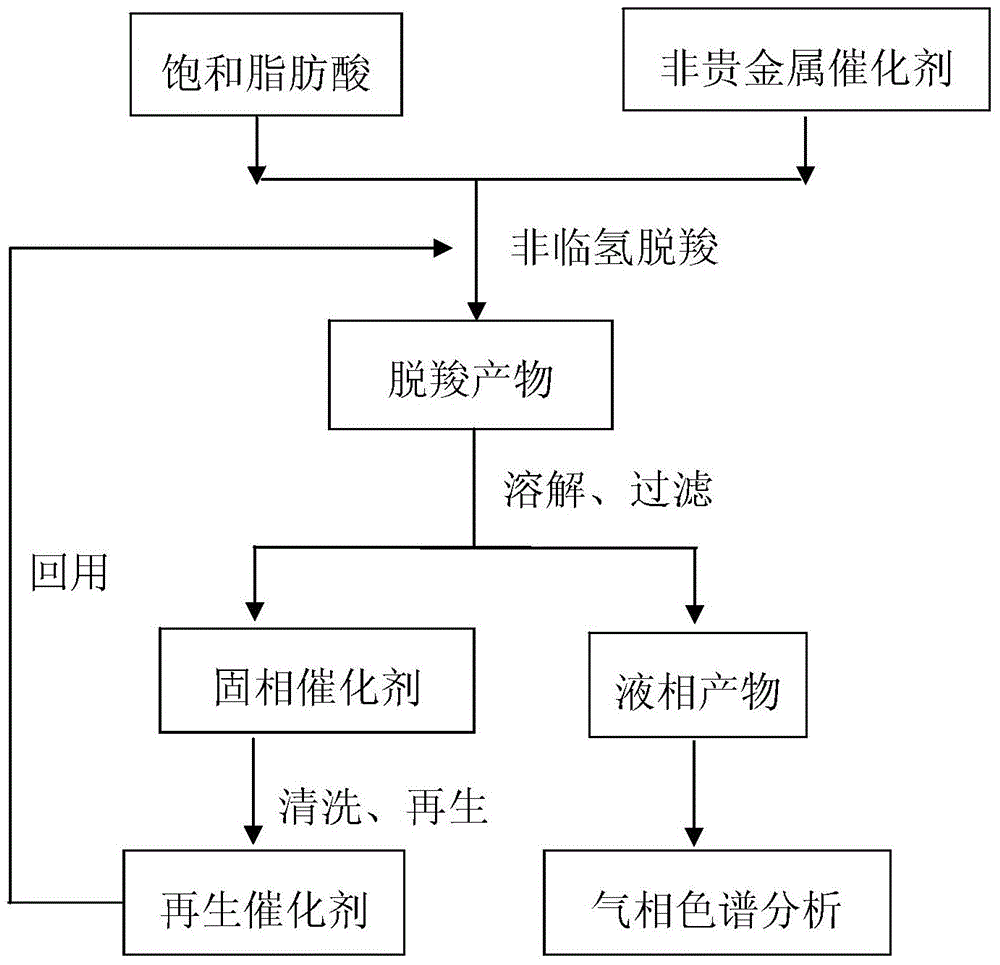
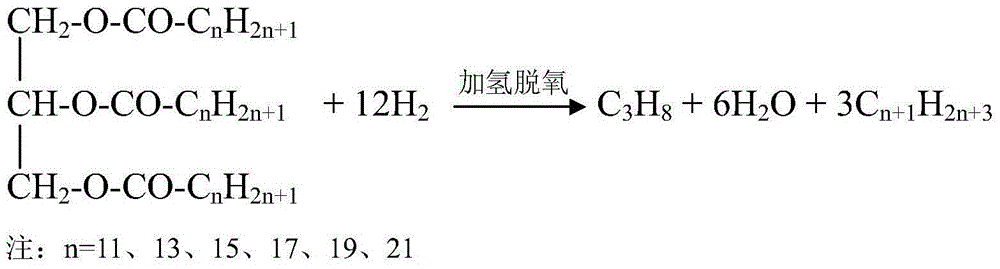
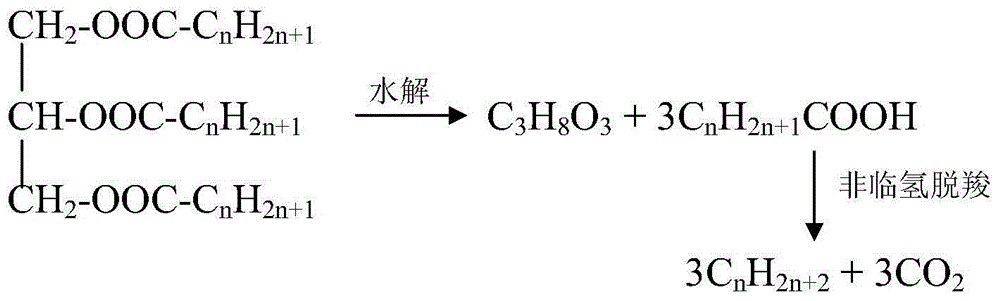
Method for using non-noble metal catalyst to catalyze decarboxylation of saturated fatty acid to prepare long-chain alkane
A non-precious metal, long-chain alkane technology, applied in the field of long-chain alkane preparation, can solve the problems of unrealistic industrialization and high cost, and achieve the effects of green production process, low hydrogen consumption and low catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 200g of lauric acid and 33.3g of Cu / ZrO with a Cu content of 30wt% in a 500mL batch autoclave 2 Stir the catalyst, heat up to 350 ° C for 5 hours for decarboxylation; after the decarboxylation reaction is completed, the decarboxylation product is cooled, dissolved with n-hexane, and filtered; the liquid phase product is fixed to volume with n-hexane and analyzed by GC-FID to calculate the moles of long-chain alkanes The yield was 32.7%.
Embodiment 2
[0030] In a 500mL batch autoclave, 200g of palmitic acid and 13.3g of Ni / ZrO with a Ni content of 20wt% were added 2 Catalyst, stir, and heat up to 330°C for 4 hours for decarboxylation; after the decarboxylation reaction is completed, the decarboxylation product is cooled, dissolved with n-hexane, and filtered; the liquid phase product is fixed to volume with acetone and analyzed by GC-FID to calculate the molar yield of long-chain alkanes The rate is 38.4%.
Embodiment 3
[0032] Add 200g of stearic acid and 22.2g of Ni / C catalyst with a Ni content of 20wt% in a 500mL batch autoclave, start stirring, and heat up to 370°C for 3 hours for decarboxylation; Dissolving and filtering; the liquid phase product was analyzed by GC-FID after constant volume with acetone, and the calculated molar yield of long-chain alkanes was 78.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com