Edible composition as well as preparation method and application thereof
The technology of a composition and medicine is applied in the field of edible composition and its preparation, which can solve the problem of in-depth adjustment of animal intestinal flora, and achieve the effects of enhancing immunity, increasing the concentration of butyric acid, and preventing or treating obesity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Preparation of inulin capsules
[0031] Weigh 10 parts by weight of inulin, process and pulverize it with ultra-fine crushing equipment to obtain fine inulin powder with a particle size of 100 microns, homogenize it into a mixed fine powder, and then place the content materials in a soft capsule pilling machine , fill and press into soft capsules according to the existing soft capsule production technology.
[0032] 2. Preparation of live Clostridium butyricum capsules
[0033] Weigh 10 parts by weight of Clostridium butyricum (ATCC product number 19398), process and pulverize it with ultra-fine grinding equipment to obtain Clostridium butyricum fine powder with a particle size of 100 microns, and homogenize it into a mixed fine powder, and then the content materials are placed in a soft capsule pellet machine, filled and compressed into soft capsules according to the existing soft capsule production technology.
[0034] 3. Preparation of Live Clostridium Butyricum...
Embodiment 2
[0069] Example 2: Function experiment of regulating human intestinal flora
[0070] 1. Grouping of feeding experiments
[0071] According to the Ministry of Public Health's "Health Food Inspection and Evaluation Technical Specifications (2003)" human body test and evaluation method for regulating intestinal flora function, each product obtained in Example 1 is evaluated, as follows:
[0072] The following is the grouping of the eating experiment:
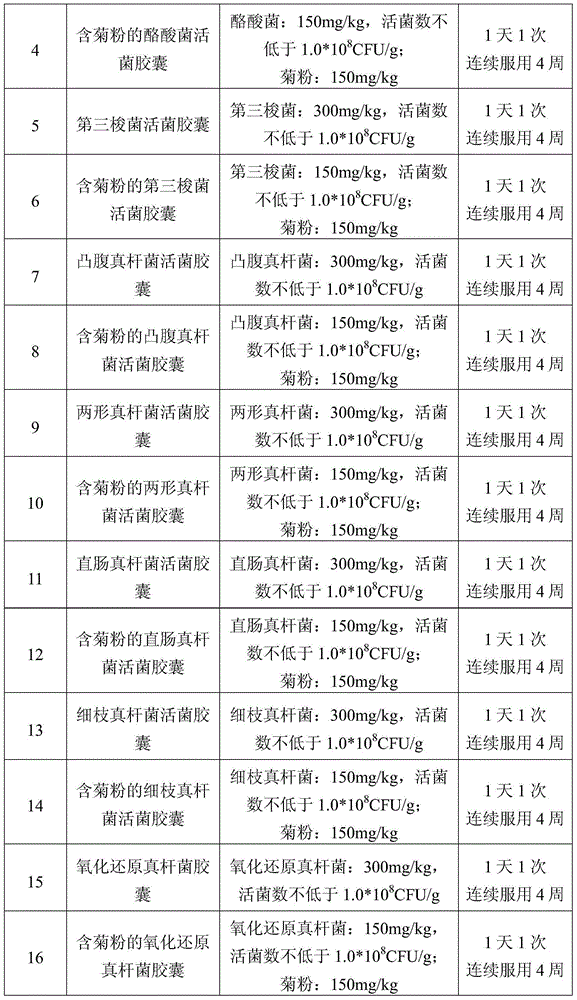
[0073] Table 1 Grouping table of eating experiment
[0074]
[0075]
[0076]
[0077] 2. Human food test method
[0078] The subjects were randomly divided into 19 groups, with 10 people in each group. Before the subjects tried to eat, the feces of the subjects were collected aseptically, and the intestinal flora was tested by 16S rDNA sequencing as a reference for the background level.
[0079] According to the grouping situation in Table 1, group 1 took live Clostridium butyricum capsules, group 2 took live Clostridiu...
Embodiment 3
[0089] Embodiment 3: hypoglycemic animal experiment
[0090] According to the animal experiment evaluation method of auxiliary hypoglycemic function of "Health Food Inspection and Evaluation Technical Specifications (2003)" of the Ministry of Health, the product obtained in Example 1 is evaluated, as follows:
[0091] 1. Test method: 190 male SD rats were taken, and a low-dose streptozotocin-induced diabetic rat model was prepared according to the standard method. After three days, the rats were randomly divided into 19 groups according to the blood sugar level, with 10 rats in each group. Each group was administered by intragastric administration for 8 consecutive days, once a day, and blood was collected two hours after the last administration to measure the blood sugar level.
[0092] 2. Test object: The tested SD rats were randomly divided into 19 groups, 10 in each group. The original diet control and activities of the tested SD rats remained unchanged. According to the g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com