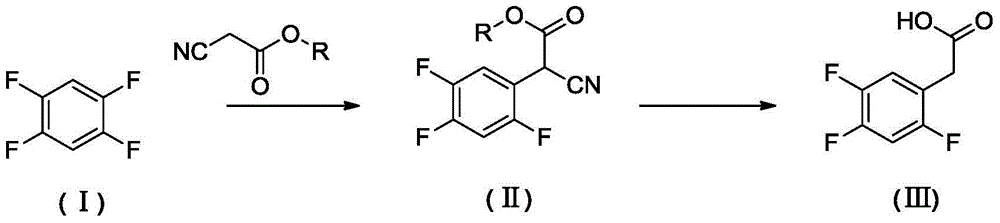
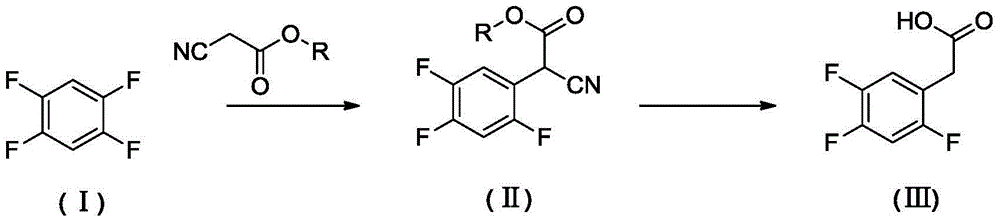
Preparation method of 2, 4,5-trifluoro phenylacetic acid
A technology of trifluorophenylacetic acid and trifluorophenyl, which is applied in the field of preparation of 2,4,5-trifluorophenylacetic acid, can solve the problems of harsh reaction conditions, high industrial production cost, and high price of oxidant, and achieves cheap, The effect of low material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of ethyl 2-cyano-2-(2,4,5-trifluorophenyl)acetate
[0026] Under nitrogen protection, add ethyl cyanoacetate (0.13mol) to N-methylpyrrolidone (30.0mL) containing sodium hydride (0.13mol, content 60%), when the gas is released, add 1,2,4 , 5-tetrafluorobenzene (0.0439mol), the reaction system was sealed, heated to 120 ° C, and reacted for 24 hours. 75 mL of water was added, extracted with ethyl acetate, and the organic phase was desolvated under reduced pressure to obtain 7.47 g of a yellow transparent liquid with a yield of 70%.
[0027] After testing, the yellow transparent liquid is ethyl 2-cyano-2-(2,4,5-trifluorophenyl)acetate, and the NMR data are as follows: 1 HNMR (400Hz, CDCl 3 ):δ7.42-7.37(m,1H),7.09-7.02(m,1H),4.96(s,1H),4.31(q,2H),1.33(t,3H).
Embodiment 2
[0028] Example 2 Preparation of ethyl 2-cyano-2-(2,4,5-trifluorophenyl)acetate
[0029] Potassium tert-butoxide (0.13mol) was used to replace sodium hydride, and the rest was the same as in Example 1. After the reaction was completed, 5.76 grams of ethyl 2-cyano-2-(2,4,5-trifluorophenyl)acetate was obtained. The yield was 54%.
Embodiment 3
[0030] Example 3 Preparation of ethyl 2-cyano-2-(2,4,5-trifluorophenyl)acetate
[0031] N,N-Dimethylacetamide (30mL) was used instead of N-methylpyrrolidone, the rest was the same as in Example 1, and the reaction was completed to obtain 2-cyano-2-(2,4,5-trifluorophenyl)acetic acid Ethyl ester 7.00 g, yield 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com