Method for synthesizing MFI type zeolite from Magadiite
A technology of zeolite and mixture, which is applied in the field of synthesizing MFI zeolite, can solve the problems of alkali addition and large amount of template agent, and achieve the effect of less dosage and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]With 0.2932 grams of NaF, 1.6762 grams of 30wt% sodium hydroxide aqueous solution, 10 grams of 50wt% PEG 300 (polyethylene glycol with an average molecular weight of about 300) solution, 10.3 grams of water and 8.1 milliliters of 40wt% silica sol The solution is mixed evenly, and the molar ratio of the resulting mixture is:
[0032] 10SiO 2 : 1.0NaF: 0.9Na 2 O: 2.5PEG 300: 175H 2 o
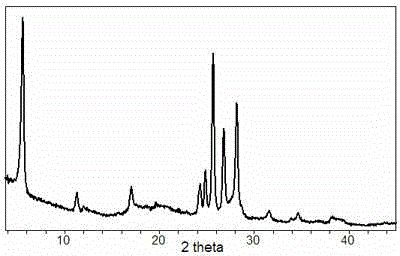
[0033] Move the above mixture into the reactor at 160 o C was crystallized for 86 hours, washed and dried after the reaction, and was identified as Magaditte material by XRD.
[0034] The above-mentioned Magadiite of 2.0 grams is mixed with 0.8 gram of TPABr and 10 milliliters of deionized water, and the molar proportion of mixed slurry is:
[0035] TPABr / SiO 2 =0.09,H 2 O / SiO 2 =16.67
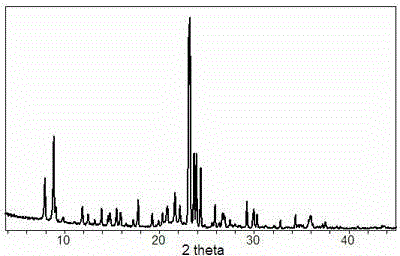
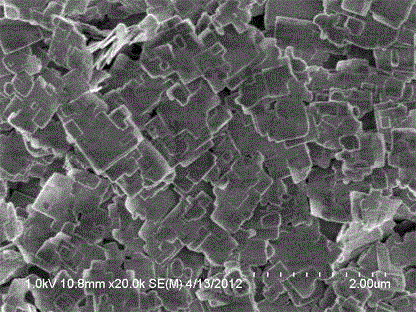
[0036] Then the above-mentioned slurry is placed in a closed reactor, at 150 o C for 40 hours of crystallization, the solid product was identified as MFI zeolite by XRD after washing and drying.
...
Embodiment 2
[0039] With 0.2932 grams of NaF, 1.6762 grams of 30wt% sodium hydroxide aqueous solution, 10 grams of 50wt% PEG 300 (polyethylene glycol with an average molecular weight of about 300) solution, 10.3 grams of water and 8.1 milliliters of 40wt% silica sol The solution is mixed evenly, and the molar ratio of the resulting mixture is:
[0040] 10SiO 2 : 1.0NaF: 0.9Na 2 O: 2.5PEG 300: 175H 2 o
[0041] Move the above mixture into the reactor at 160 o C was crystallized for 86 hours, washed and dried after the reaction, and was identified as Magaditte material by XRD.
[0042] The above-mentioned Magadiite of 2.0 grams is mixed with 0.8 gram of TPABr and 10 milliliters of deionized water, and the molar proportion of mixed slurry is:
[0043] TPABr / SiO 2 =0.09,H 2 O / SiO 2 =16.67
[0044] Then the above-mentioned slurry is placed in a closed reactor, at 130 o C for 60 hours of crystallization, the solid product was identified as MFI zeolite by XRD after washing and drying. ...
Embodiment 3
[0047] With 0.2932 grams of NaF, 1.6762 grams of 30wt% sodium hydroxide aqueous solution, 10 grams of 50wt% PEG 300 (polyethylene glycol with an average molecular weight of about 300) solution, 10.3 grams of water and 8.1 milliliters of 40wt% silica sol The solution is mixed evenly, and the molar ratio of the resulting mixture is:
[0048] 10SiO 2 : 1.0NaF: 0.9Na 2 O: 2.5PEG 300: 175H 2 o
[0049] Move the above mixture into the reactor at 160 o C was crystallized for 86 hours, washed and dried after the reaction, and was identified as Magaditte material by XRD.
[0050] The above-mentioned Magadiite of 2.0 grams is mixed with 0.8 gram of TPABr and 10 milliliters of deionized water, and the molar proportion of mixed slurry is:
[0051] TPABr / SiO 2 =0.09,H 2 O / SiO 2 =16.67
[0052] Then the above-mentioned slurry is placed in a closed reactor, at 180 o C for 24 hours of crystallization, the solid product was identified as MFI zeolite by XRD after washing and drying. ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap