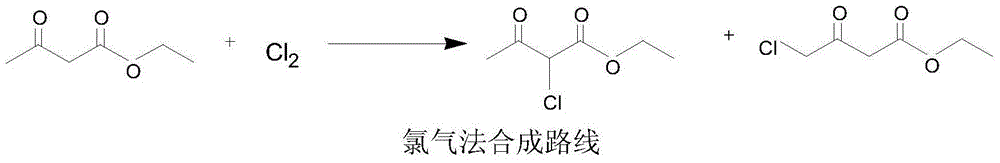
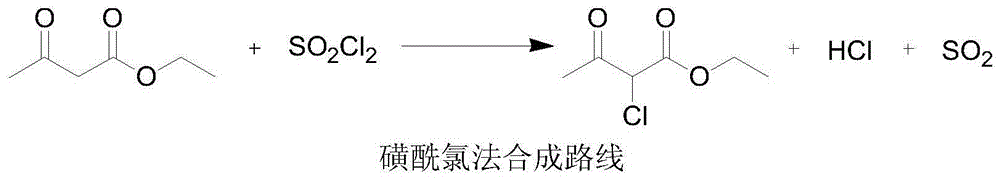
Preparation method of 2-chloroacetoacetic acid ethyl ester
A technology of ethyl chloroacetoacetate and ethyl acetoacetate, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as large amount of waste water, low yield of ethyl 2-chloroacetoacetate, and inability to treat waste COD acid, and achieve The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 130kg of ethyl acetoacetate to the reaction kettle, add 135kg of sulfuryl chloride (1.03 times) dropwise within 2 hours at room temperature, keep the temperature in the chlorination kettle at 80-100°C during the dropping process, and keep the vacuum at -0.3-0.35MPa , the hydrogen chloride and sulfur dioxide gases generated in the reaction are introduced into the oxidation deacidification tower by vacuum, and the oxidation deacidification tower is turned on to spray, the oxidation temperature is controlled at 55-65°C, the pressure is -0.02--0.05MPa, and 400kg (3.1 times, 20 %) sulfur dioxide generated by the oxidation of hydrogen peroxide. After the sulfuryl chloride was added dropwise, the chlorination kettle was kept at 80-100°C for 2 hours. After the heat preservation was completed, 360kg (10.1%) of hydrochloric acid and 140kg (69.8%) of sulfuric acid were distilled at 100-110°C. After the crude product was rectified, 153.8 kg of ethyl 2-chloroacetoacetate was obt...
Embodiment 2
[0026] Add 130kg of ethyl acetoacetate to the reaction kettle, add 149kg of sulfuryl chloride (1.15 times) dropwise within 2 hours at room temperature, keep the temperature in the chlorination kettle at 80-100°C during the dropping process, and keep the vacuum at -0.3-0.5MPa , the hydrogen chloride and sulfur dioxide gases generated in the reaction are introduced into the oxidation deacidification tower by vacuum, and the oxidation deacidification tower is turned on to spray, the oxidation temperature is controlled at 45-60°C, the pressure is -0.02--0.05MPa, and 300kg (2.3 times, 20 %) sulfur dioxide generated by the oxidation of hydrogen peroxide. After the sulfuryl chloride was added dropwise, the chlorination kettle was kept at 80-100°C for 1 hour. After the heat preservation was completed, 240kg (15.2%) of hydrochloric acid and 158kg (62%) of sulfuric acid were distilled at 100-110°C. After the crude product was rectified, 153kg of ethyl 2-chloroacetoacetate was obtained, ...
Embodiment 3
[0028] Add 130kg of ethyl acetoacetate to the reaction kettle, add 140kg (1.07 times) of sulfuryl chloride dropwise within 2 hours at room temperature, keep the temperature in the chlorination kettle at 80-100°C during the dropping process, and keep the vacuum at -0.4-0.5MPa , the hydrogen chloride and sulfur dioxide gases generated in the reaction are introduced into the oxidation deacidification tower by vacuum, and the oxidation deacidification tower is turned on to spray, the oxidation temperature is controlled at 65-75 °C, the pressure is -0.02--0.05MPa, and 65kg (0.5 times, 20 %) sulfur dioxide generated by the oxidation of hydrogen peroxide. After the sulfuryl chloride was added dropwise, the chlorination kettle was kept at 80-100°C for 2 hours. After the heat preservation was completed, 180kg (20.3%) of hydrochloric acid and 116kg (84.5%) of sulfuric acid were distilled at 100-110°C. After the crude product was rectified, 155.1 kg of ethyl 2-chloroacetoacetate was obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com