Method for detecting content of heavy metals in plant sample
A technique for heavy metals and samples, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The pretreatment of embodiment 1 vegetable sample
[0031] Chemical reagents:
[0032] Potassium dichromate (spectrum pure, purchased from Beijing Chemical Plant);
[0033] Manganese sulfate (analytically pure, purchased from Beijing Chemical Plant);
[0034] Zinc sulfate (analytically pure, purchased from Beijing Chemical Plant);
[0035] Cadmium sulfate (analytically pure, purchased from Beijing Chemical Plant);
[0036] Copper sulfate (analytically pure, purchased from Beijing Chemical Plant);
[0037] Concentrated hydrochloric acid (analytically pure, purchased from Beijing Chemical Plant);
[0038] Concentrated nitric acid (analytically pure, purchased from Beijing Chemical Plant);
[0039] Perchloric acid (excellent grade, purchased from Beijing Chemical Plant).
[0040] Vegetable samples:
[0041] Fresh Chinese cabbage (non-heading Chinese cabbage, the young plant of Brassica chinensis L.), was purchased randomly from Erli Street Vegetable Market in Hefei. ...
Embodiment 2
[0059] Embodiment 2: Atomic absorption spectroscopic analysis of vegetable samples
[0060] The atomic absorption spectrophotometer used is American Thermo Elenent SOLAAR M5 type.
[0061] Treat the sample with an atomic absorption spectrometer, flame type: air-acetylene, and the conditions are as follows
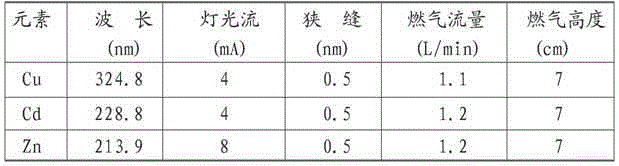
[0062] Table 1: Element instrument measurement conditions
[0063]
[0064] Prepare Cu, Zn, and Cd 1000 μg / mL standard stock solutions respectively.
[0065] Standard solution preparation is carried out according to the following method:
[0066] (1) Cu Weigh 0.5000g of metal Cu (high purity) and dissolve it in a small amount of nitric acid, evaporate to dryness on a water bath, add 5ml hydrochloric acid and evaporate to dryness, dissolve and dilute to 500ml with 1mol / L hydrochloric acid, then 1000mg / L Cu standard stock solution.
[0067] (2) Zn: Weigh 0.5000g of metal Zn(AR), dilute it to 500ml with a small amount of 6N hydrochloric acid, and obtain a 1000mg / L sta...
Embodiment 3
[0083] Embodiment 3: dilute acid extraction method and national standard method recovery rate comparison
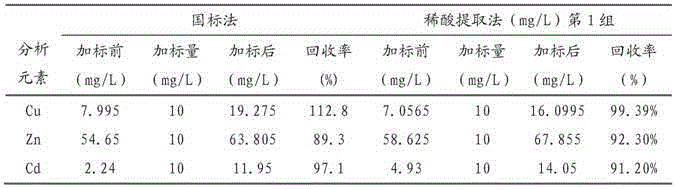
[0084] Add a certain known concentration of each heavy metal solution to the unknown sample, measure the concentration of the heavy metal before and after the standard addition respectively, and calculate the recovery rate of the method, that is, the recovery rate=C 加标后 -C 加标前 ) / C 加标量 ×100%, the results are shown in Table 5. The standard addition recovery experiment is carried out according to the following method: get two parts of equal amount of vegetable samples (prepared according to the method of Example 1), wherein a certain amount of standard stock solution Cu, Zn, Cd is added (prepared according to the method of Example 2) , the other part was not added, and the sample was processed in the same way. The heavy metal content was measured, the recovery rate was calculated, and the recovery rates of the national standard method and the dilute acid method (perform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com