A kind of preparation method of rivaroxaban intermediate
A technology for rivaroxaban and intermediates, which is applied in the field of anticoagulant rivaroxaban intermediates and its preparation, can solve problems such as inability to industrialize, and achieve the effects of simple operation, less waste, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of N-Hydroxyethylaniline (Ⅱ)
[0054] Add 21.2mL (0.20mol) of bromobenzene, 18.5mL (0.30mol) of ethanolamine, 4.0g (0.02mol) of cuprous iodide, and 55.2g of potassium carbonate into a 250mL four-necked flask equipped with mechanical stirring, a condenser, and a thermometer. (0.4mol), water 100mL, temperature controlled 80°C and stirred for 15h. After suction filtration, the filtrate was rectified to obtain 25.2 g of a colorless liquid (boiling point 165° C. at 10 mmHg), which was the target compound N-hydroxyethylaniline (II), with a yield of 91.1%.
Embodiment 2
[0056] Preparation of 4-nitroso-N-hydroxyethylaniline (Ⅲ)
[0057] Add 87.6g of 10% hydrochloric acid into a 500mL four-necked bottle equipped with a mechanical stirrer and a thermometer, cool to 5°C, add 29g (0.21mol) of N-hydroxyethylaniline (II), and add 58g of 30 % sodium nitrite aqueous solution (0.25mol), dropwise for about 3 hours, after dropping, keep warm for 2 hours, filter, wash the filter cake twice with an appropriate amount of water, and dry with hot air at 60°C to obtain 31.7g of yellow solid with a yield of 90.2%.
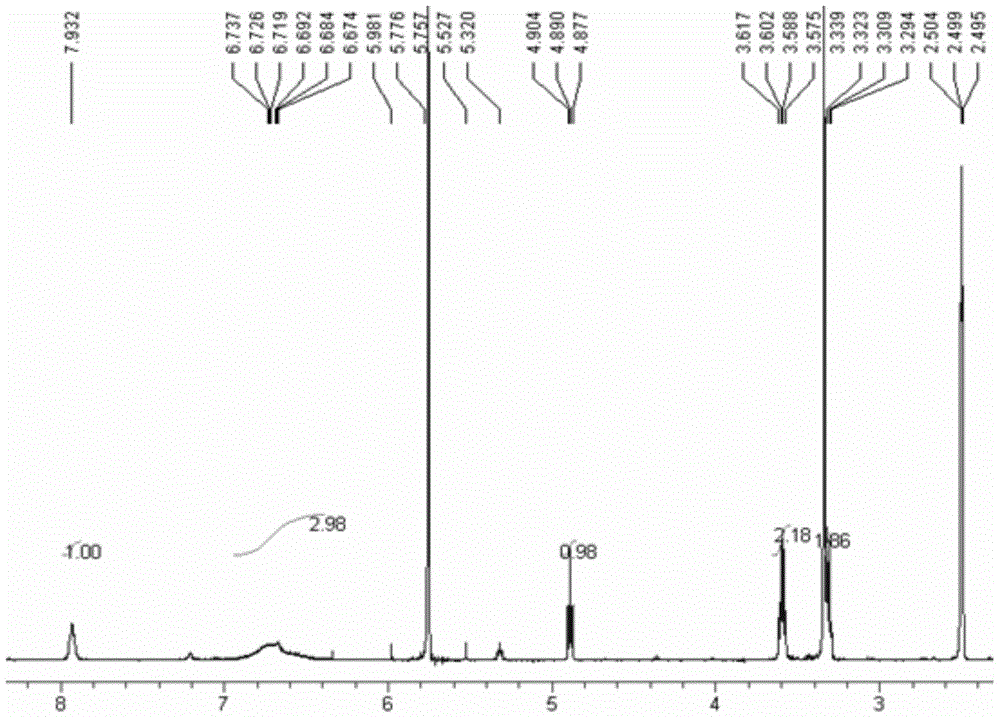
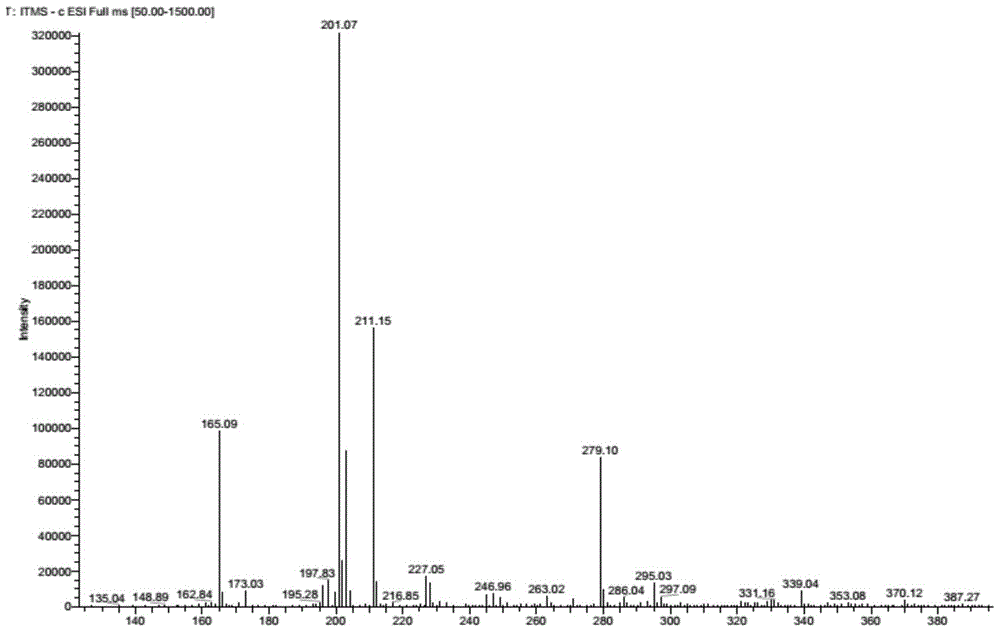
[0058] III: 1 H NMR (300MHz, DMSO-d 6 ):δ7.92(s,1H),6.67-6.74(br,3H),4.89(t,1H,J=4.2Hz),3.58(m,2H),3.30(m,2H); ESI-MS m / z=165(M-1), 201(M+Cl - ).
Embodiment 3
[0060] Preparation of 4-nitroso-N-hydroxyethylaniline (Ⅲ)
[0061] Add 87.6g of 10% hydrochloric acid into a 500mL four-necked bottle equipped with a mechanical stirrer and a thermometer, cool to 5°C, add 29g (0.21mol) N-hydroxyethylaniline (II), and add 58g dropwise at a temperature control of ≤ -10°C 30% aqueous solution of sodium nitrite (0.25mol), the time of dropping is about 2.5h, after dropping, keep warm for 6h, filter, wash the filter cake twice with an appropriate amount of water, and dry it with hot air at 60°C to obtain 30.2g of yellow solid, with a yield of 86.0% .
[0062] III: 1 H NMR (300MHz, DMSO-d 6 ):δ7.92(s,1H),6.67-6.74(br,3H),4.89(t,1H,J=4.2Hz),3.58(m,2H),3.30(m,2H); ESI-MS m / z=165(M-1), 201(M + Cl - ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com