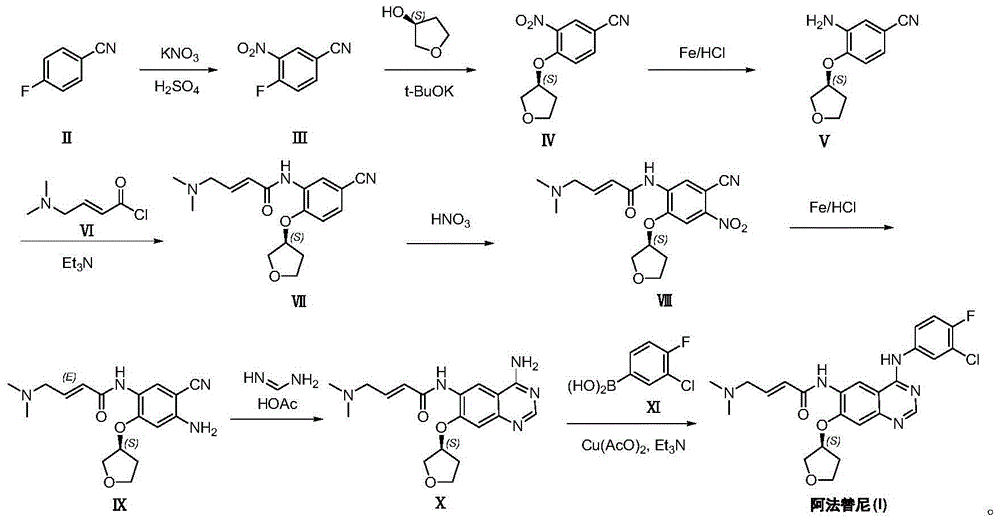
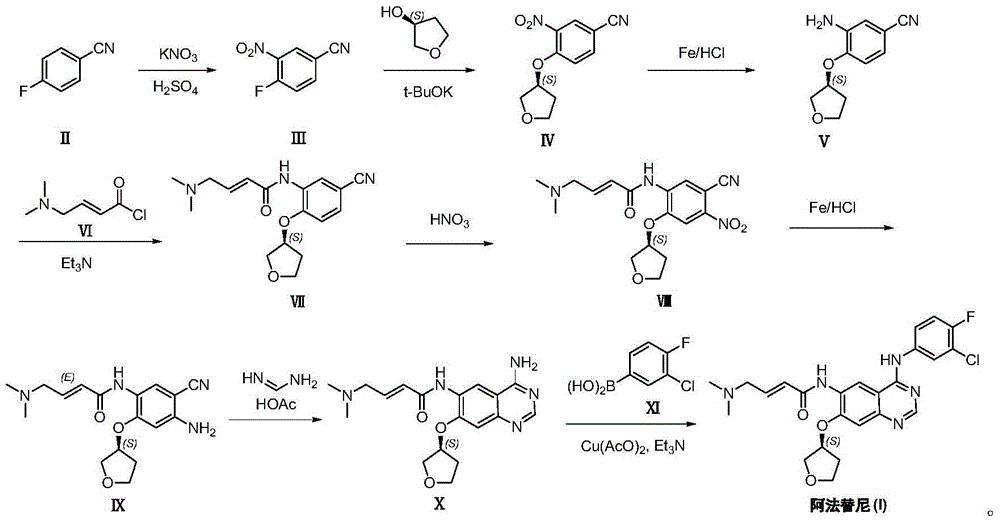
Preparation method of Afatinib
A technology of afatinib and tetrahydrofuran, which is applied in the field of preparation of raw materials and can solve the problem of high cost of afatinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Preparation of 3-nitro-4-fluorobenzonitrile (Ⅲ): Dissolve 4-fluorobenzonitrile (25g, 206mmol) in concentrated sulfuric acid (125mL), and add to the system in batches under temperature control at 0°C Potassium nitrate (20.8g, 206mmol) was added, reacted at 0°C for 30 minutes, and extracted twice with dichloromethane, each with 100mL. The organic phases were combined, washed with water (60 mL), dried, and concentrated to obtain 20 g of white solid product (III), yield: 58%. NMR, CDCl 3 ,400MHz, δ8.42(1H,d),7.88(1H,dd),7.41(1H,d).
[0025] (2) Preparation of 3-nitro-4-[(S)-(tetrahydrofuran-3-yl)oxy]benzonitrile (IV): Potassium tert-butoxide (16.8g, 150mmol), (S)-tetrahydrofuran -3-alcohol (8.8g, 100mmol) and 3-nitro-4-fluorobenzonitrile (Ⅲ) (16.6g, 100mmol) were mixed in DMF (100mL), heated to 60°C for 5 hours; cooled to room temperature, Add 100 mL of water to quench the reaction; extract twice with dichloromethane, each with 100 mL; combine the organic phases, was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com