Osteogenic differentiation of mesenchymal stem cells
A technology of osteogenic differentiation and mesenchymal stem cells, applied in animal cells, vertebrate cells, cell culture active agents, etc., can solve the problem of poor understanding of recipient cell differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0060] General description of the process
[0061] Isolation and culture of monocytes: Monocytes were isolated from human blood using magnetic separation and cultured on different biomaterials with or without stimulation (eg LPS). Mesenchymal stem cells are obtained from human bone marrow by gradient separation. After 72 h, cells were harvested and exosomes were isolated from conditioned media.
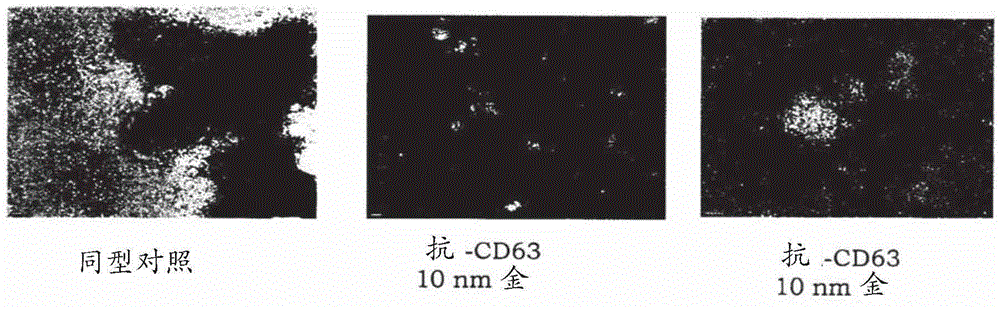
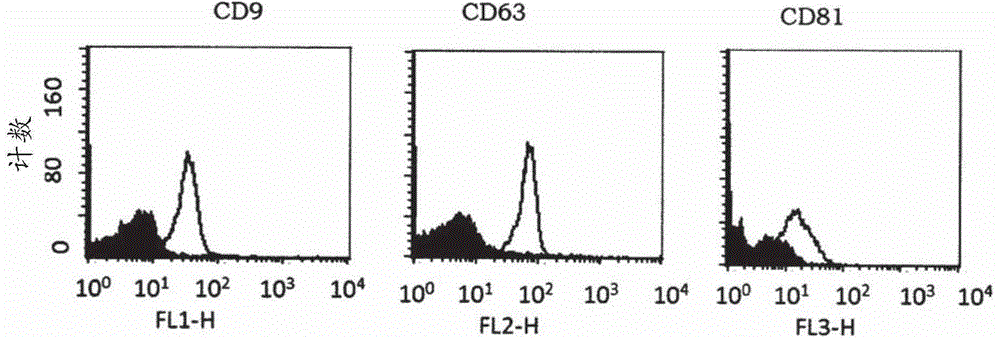
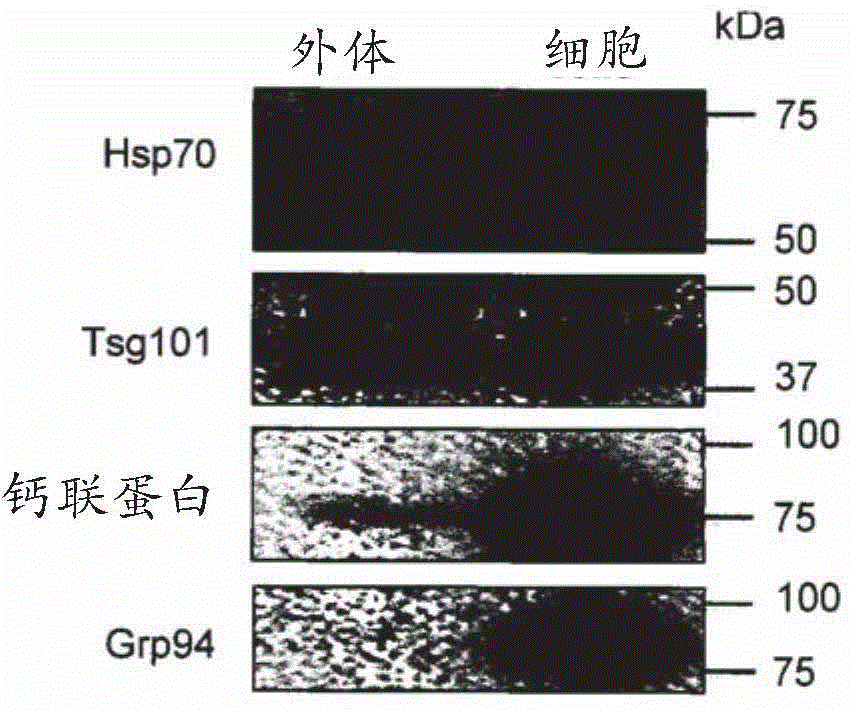
[0062] Exosome Isolation and Detection: Exosomes will be isolated using a method based on repeated centrifugation and filtration steps to remove cellular debris, apoptotic bodies, etc., followed by ultracentrifugation to pellet exosomes. Alternative separation methods can also be used. Exosomes will be detected using a combination of methods including electron microscopy as well as detection of markers commonly found on exosomes (e.g., CD9, CD63, CD81, Tsg101) and a marker that should not be present in exosomes (calnexin).
[0063] mRNA and microRNA content of exosomes: Microarray...
example 2
[0074] Human primary LPS-stimulated monocytes. After 3 days in culture, exosomes were isolated and RNA was extracted. Bioanalyzer analysis of cellular and exosomal total RNA and small RNA. The electropherogram shows ( Figure 4 ) (a) Nucleotide size distribution (nt) and fluorescence intensity (FU) of total RNA in exosomes and cells (b) and small RNAs in exosomes (c) and cells (d).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com