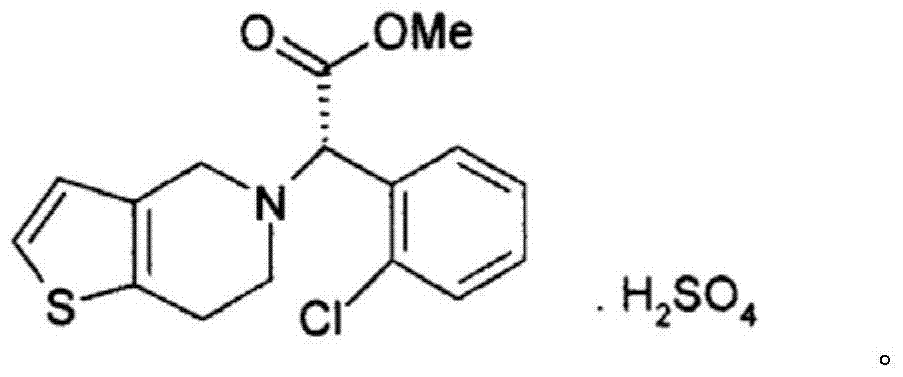
I type clopidogrel hydrogen sulfate particles and preparation method thereof as well as I type clopidogrel hydrogen sulfate solid preparation and preparation method thereof
A technology of clopidogrel hydrogen sulfate and granules, applied in the directions of pill delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve problems such as instability of type I clopidogrel hydrogen sulfate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The invention provides a preparation method of type I clopidogrel bisulfate granules, comprising the following steps:
[0039] a), the crystal form stabilizer is mixed with a solvent to obtain a crystal form stabilizer solution; type I clopidogrel hydrogen sulfate is mixed with auxiliary materials to obtain a mixture;
[0040] The crystal form stabilizer is one or more of polyvinylpyrrolidone, copovidone and polyethylene glycol; the auxiliary material is a filler and / or a disintegrant;
[0041] b), the crystal form stabilizer solution and the mixture are sequentially mixed, granulated and dried to obtain type I clopidogrel hydrogen sulfate granules.
[0042] In the preparation method of type I clopidogrel bisulfate granules provided by the present invention, firstly, the crystal form stabilizer is mixed with a solvent to obtain a crystal form stabilizer solution. Wherein, the crystal form stabilizer is one or more of polyvinylpyrrolidone, copovidone and polyethylene gl...
Embodiment 1
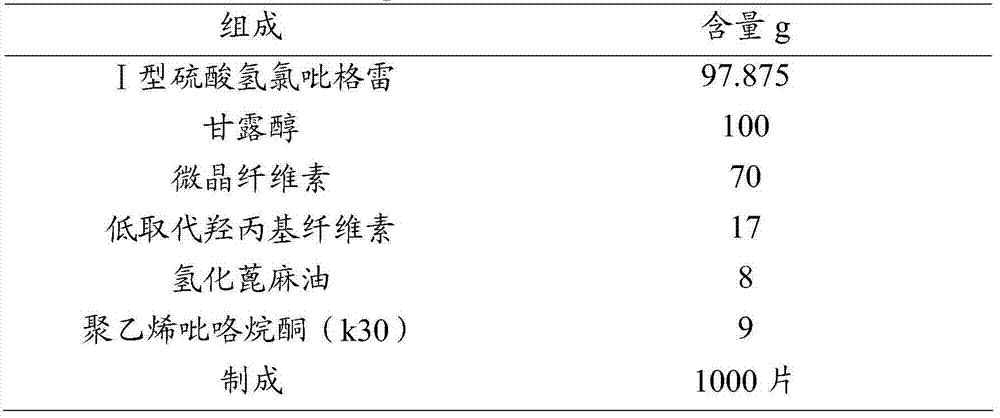
[0090] Prescription: Clopidogrel 75 mg / tablet
[0091]
[0092] Preparation Process:
[0093] 1) Mix 97.875g of clopidogrel hydrogen sulfate type I, 100g of mannitol, 70g of microcrystalline cellulose, and 17g of low-substituted hydroxypropyl cellulose to obtain a mixed material.
[0094] 2) Dissolving 9 g of polyvinylpyrrolidone (k30) in a small amount of ethanol to obtain a polyvinylpyrrolidone solution.
[0095] 3) Add polyvinylpyrrolidone solution to the mixed material to prepare soft material, which is granulated through a 24-mesh sieve in a swing granulator.
[0096] 4) The prepared granules were dried at 60° C. for 2 hours, then passed through a 18-mesh sieve for granulation, and 8 g of hydrogenated castor oil was added to the granulated granules, mixed evenly, and tableted to obtain a clopidogrel content of 75 mg / Tablets of 1000 tablets.
Embodiment 2
[0098] Prescription: Clopidogrel 75 mg / tablet
[0099]
[0100] Preparation Process:
[0101] 1) Mix 97.875g of clopidogrel hydrogen sulfate type I, 60g of mannitol, 120g of microcrystalline cellulose, and 12g of low-substituted hydroxypropyl cellulose to obtain a mixed material.
[0102] 2) Dissolving 6 g of polyvinylpyrrolidone (k90) in a small amount of ethanol to obtain a polyvinylpyrrolidone solution.
[0103] 3) Add polyvinylpyrrolidone solution to the mixed material to prepare soft material, which is granulated through a 24-mesh sieve in a swing granulator.
[0104] 4) The prepared granules were dried at 60° C. for 2 hours, then passed through a 18-mesh sieve for granulation, and 6 g of hydrogenated castor oil was added to the granulated granules, mixed evenly, and pressed into tablets to obtain a clopidogrel content of 75 mg / Tablets of 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com