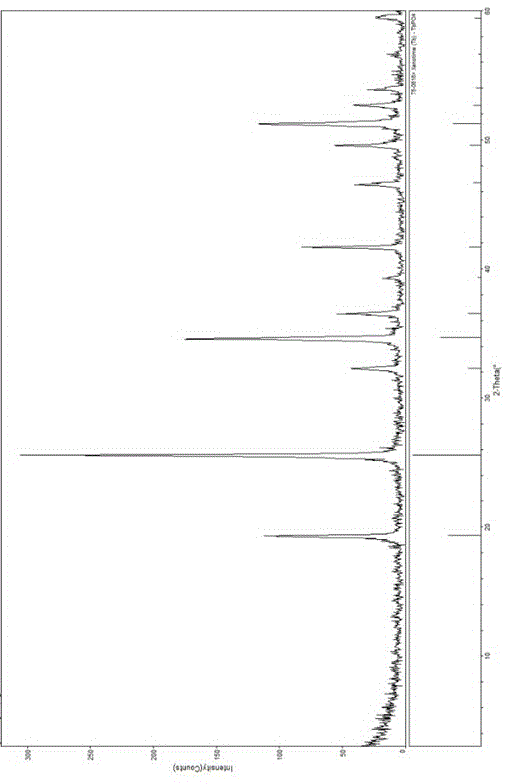
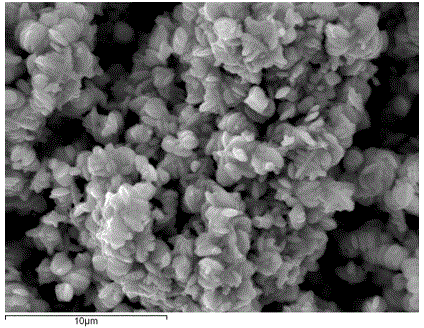
Method for CO2-enriched hydro-thermal synthesis of flower-shaped terbium phosphate
A technology of hydrothermal synthesis and terbium phosphate, which is applied in the field of preparation of rare earth phosphate materials, can solve the problems of not very high crystallinity, not very uniform size, lack of crystal morphology and the like, and achieves uniform size, good crystallinity and environmental friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Weigh a certain mass of terbium nitrate [Tb(NO 3 ) 3 ·6H 2 O] and diammonium hydrogen phosphate [(NH 4 ) 2 HPO 4 ], and deionized water was used to make an aqueous solution of terbium nitrate and an aqueous solution of diammonium hydrogen phosphate with a concentration of 0.36mol / L respectively. The terbium nitrate solution and the diammonium hydrogen phosphate solution were added into the reaction kettle according to the volume ratio of 1:1, and stirred while adding, and the initial filling degree of the reaction kettle was controlled to be 70%, and the stirring was continued for 30 min to 1 h. Fill the autoclave with 1 MPa carbon dioxide gas and close the autoclave. The autoclave was heated to control the reaction temperature at 180 °C for 2 days, and then the autoclave was naturally cooled to room temperature. After the reaction kettle was depressurized, the product was filtered, centrifuged, and washed twice with deionized water and absolute ethanol alternatel...
Embodiment 2
[0016] Weigh a certain mass of terbium nitrate [Tb(NO 3 ) 3 ·6H 2 O] and diammonium hydrogen phosphate [(NH 4 ) 2 HPO 4 ], and deionized water was used to make an aqueous solution of terbium nitrate and an aqueous solution of diammonium hydrogen phosphate with a concentration of 0.36mol / L respectively. The terbium nitrate solution and the diammonium hydrogen phosphate solution were added into the reaction kettle according to the volume ratio of 1:1, and stirred while adding, and the initial filling degree of the reaction kettle was controlled to be 70%, and the stirring was continued for 30 min to 1 h. Fill the autoclave with 5 MPa carbon dioxide gas and close the autoclave. The autoclave was heated to control the reaction temperature at 180 °C for 2 days, and then the autoclave was naturally cooled to room temperature. After the reaction kettle was depressurized, the product was filtered, centrifuged, and washed twice with deionized water and absolute ethanol alternatel...
Embodiment 3
[0018] Weigh a certain mass of terbium nitrate [Tb(NO 3 ) 3 ·6H 2 O] and diammonium hydrogen phosphate [(NH 4 ) 2 HPO 4 ], and deionized water was used to make an aqueous solution of terbium nitrate and an aqueous solution of diammonium hydrogen phosphate with a concentration of 0.36mol / L respectively. The terbium nitrate solution and the diammonium hydrogen phosphate solution were added into the reaction kettle according to the volume ratio of 1:1, and stirred while adding, and the initial filling degree of the reaction kettle was controlled to be 70%, and the stirring was continued for 30 min to 1 h. Add dry ice (solid carbon dioxide) to the autoclave, control the pressure inside the autoclave to 10 Mpa, and close the autoclave. The autoclave was heated to control the reaction temperature at 180 °C for 2 days, and then the autoclave was naturally cooled to room temperature. After the reaction kettle was depressurized, the product was filtered, centrifuged, and washed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com