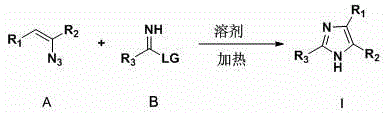
Preparation method polysubstituted imidazole derivatives
A polysubstituted and derivative technology is applied in the field of preparation of polysubstituted imidazole derivatives, which can solve the problems of complicated synthesis steps, harsh reaction conditions, limited substituents, etc., and achieves easy availability of raw materials, simple operation, and cheap reaction raw materials. easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 2,4-Diphenyl-5-carbonyl-1 H - Preparation of imidazole
[0029] 1. Raw material 1: preparation of ethyl benzimide
[0030] Add 3.43ml (33.6mmol) of benzonitrile and 15.7ml (33.6mmol, 1.0eq.) of ethanol to the three-necked flask, slowly add 18.7ml (33.6mmol, 1.0eq.) of acetyl chloride dropwise under ice-bath conditions, After the addition was complete, it was stirred overnight at room temperature. After the disappearance of the raw materials detected by the TLC plate, the reaction system was extracted with ethyl acetate, and the solvent was evaporated under reduced pressure to obtain a light yellow liquid, which was directly used in the next reaction with a yield of 92%.
[0031] 2. Raw material 2: Preparation of ethyl 2-azido-3-phenylacrylate
[0032] Add sodium 713mg (31mmol) slowly to a three-necked bottle filled with 20ml of ethanol to make sodium ethoxide. After returning to room temperature, transfer to a low temperature environment of -15°C, slowl...
Embodiment 2
[0038] Example 2: 2-Phenyl-4-methoxyphenyl-5-carbonyl-1 H - Preparation of imidazole
[0039] The operation process is the same as in Example 1, except that ethyl 2-azido-3-methoxyphenylacrylate is used instead of ethyl 2-azido-3-phenylacrylate, and the reaction is subjected to silica gel column chromatography to obtain a yellow solid. The rate is 76%, melting point: 122.9-123.3 ℃.
[0040] Its structural formula is:
[0041]
[0042] 1 H NMR (500 MHz, CDCl 3 ) δ 10.18 (s, 1H), 7.97 (m, 3H), 7.48 -7.36 (m, 6H), 4.35 (q, J = 7.1 Hz, 2H), 1.69 (s, 3H), 1.33 (t, J = 7.1 Hz, 3H); 13 C NMR (125 MHz, CDCl 3 for C 19 h19 N 2 o 3 [M+H] + = 323.1390, found 323.1394.
Embodiment 3
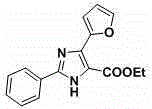
[0043] Example 3: 2-Phenyl-4-(2-furan)-5-carbonyl-1 H - Preparation of imidazole
[0044] The operation process is the same as in Example 1, except that ethyl 2-azido-3-(2-furan)acrylate is used instead of ethyl 2-azido-3-phenylacrylate, and the reaction is subjected to silica gel column chromatography to obtain a yellow solid, which is collected as The rate is 52%, and the melting point is 117.7-118.0°C.
[0045] Its structural formula is:
[0046]
[0047] 1 H NMR (500 MHz, CDCl 3 ) δ 10.19 (s, 1H), 7.98 (m, 3H), 7.44 (m, 5H), 4.35 (q, J = 7.1 Hz, 2H), 1.33 (t, J = 7.1 Hz, 3H); 13 C NMR (125 MHz, CDCl 3 for C 16 h 15 N 2 o 3 [M+H] + = 283.1077, found 283.1098.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com