Quetiapine fumarate sustained-release capsule and preparation method thereof
A technology for quetiapine fumarate and quinoline fumarate, which is applied in the field of pharmaceutical preparations and can solve the problems of complex process, many preparation steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
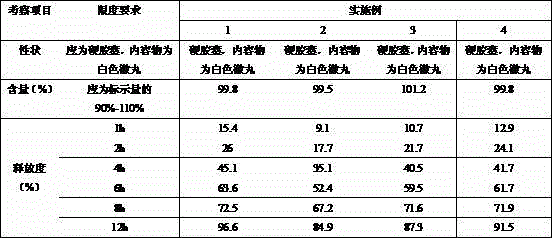
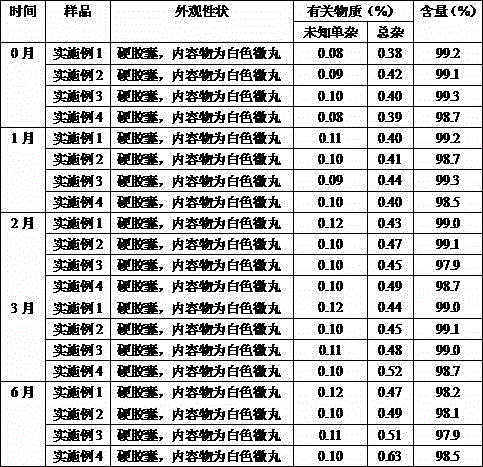
[0018] Example 1 Preparation of quetiapine fumarate sustained-release capsules (calculated as quetiapine, 50 mg)
[0019] Bead composition Dosage g Quetiapine Fumarate 58 Ethyl cellulose 20 Hypromellose 20 microcrystalline cellulose 110 Povidone K30 37 talcum powder 5 total 250
[0020] Preparation Process:
[0021] (1) Take the prescribed amount of quetiapine fumarate, ethyl cellulose, microcrystalline cellulose, povidone K30, and talcum powder, mix well, pass through an 80-mesh sieve and mix well;
[0022] (2) Prepare 3% hydroxypropyl methylcellulose aqueous solution as binder;
[0023] (3) Add appropriate amount of (2) to (1) to prepare soft material;
[0024] (4) Put the above-mentioned soft material into the extrusion spheronizer, extrude in a strip state, the extrusion mesh is 20 mesh, put the extruded strip in the centrifugal spheronizer, adjust the suitable speed of 200rpm-600rpm, and spheronize;
[0025] (5) ...
Embodiment 2
[0027] Example 2 Preparation of quetiapine fumarate sustained-release capsules (calculated as quetiapine, 200 mg)
[0028] Bead composition Dosage g Quetiapine Fumarate 230 Ethyl cellulose 50 Hypromellose 16.5 microcrystalline cellulose 53.5 Povidone K30 6 talcum powder 24 total 380
[0029] Preparation Process:
[0030] (1) Take the prescribed amount of quetiapine fumarate, ethyl cellulose, hydroxypropyl methyl cellulose, microcrystalline cellulose, and talcum powder, mix them evenly, and pass through an 80-mesh sieve to mix evenly;
[0031] (2) prepare 3% povidone K30 aqueous solution as adhesive;
[0032] (3) Add appropriate amount of (2) to (1) to prepare soft material;
[0033] (4) Put the above-mentioned soft material into the extrusion spheronizer, extrude in a strip state, the extrusion mesh is 20 mesh, put the extruded strip in the centrifugal spheronizer, adjust the suitable speed of 200rpm-600rpm, and sphero...
Embodiment 3
[0036] Example 3 Preparation of quetiapine fumarate sustained-release capsules (calculated as quetiapine, 300 mg)
[0037] Bead composition Dosage g Quetiapine Fumarate 345 Ethyl cellulose 86.25 Hypromellose 86.25 microcrystalline cellulose 103.5 Povidone K30 2.1 silica 6.9 total 630
[0038] (1) Take the prescribed amount of quetiapine fumarate, ethyl cellulose, microcrystalline cellulose, povidone K30, and talcum powder, mix well, pass through an 80-mesh sieve and mix well;
[0039] (2) Prepare 3% hydroxypropyl methylcellulose aqueous solution as binder;
[0040] (3) Add appropriate amount of (2) to (1) to prepare soft material;
[0041] (4) Put the above-mentioned soft material into the extrusion spheronizer, extrude in a strip state, the extrusion mesh is 20 mesh, put the extruded strip in the centrifugal spheronizer, adjust the suitable speed of 200rpm-600rpm, and spheronize;
[0042] (5) Dry in a fluidized bed un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com