Method for preparing sulfur auto-doped titanium dioxide photocatalyst
A technology of titanium dioxide and photocatalyst, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of pollution, environmental protection, low and poor activity of sulfur-doped titanium dioxide photocatalysts, etc., to achieve Simplified preparation process, stable and efficient photocatalytic activity, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 0.1 g TiS 2 , it was slowly added to 18 grams of deionized aqueous solution, fully stirred and mixed evenly. Then the mixed solution was put into a 20ml high-pressure reaction kettle, sealed and placed in a muffle furnace, and reacted hydrothermally at 120° C. for 4 hours. After the reaction is finished, it is naturally cooled to room temperature, washed and separated, and then dried at a low temperature to obtain a light yellow sulfur self-doped titanium dioxide nanometer photocatalyst.
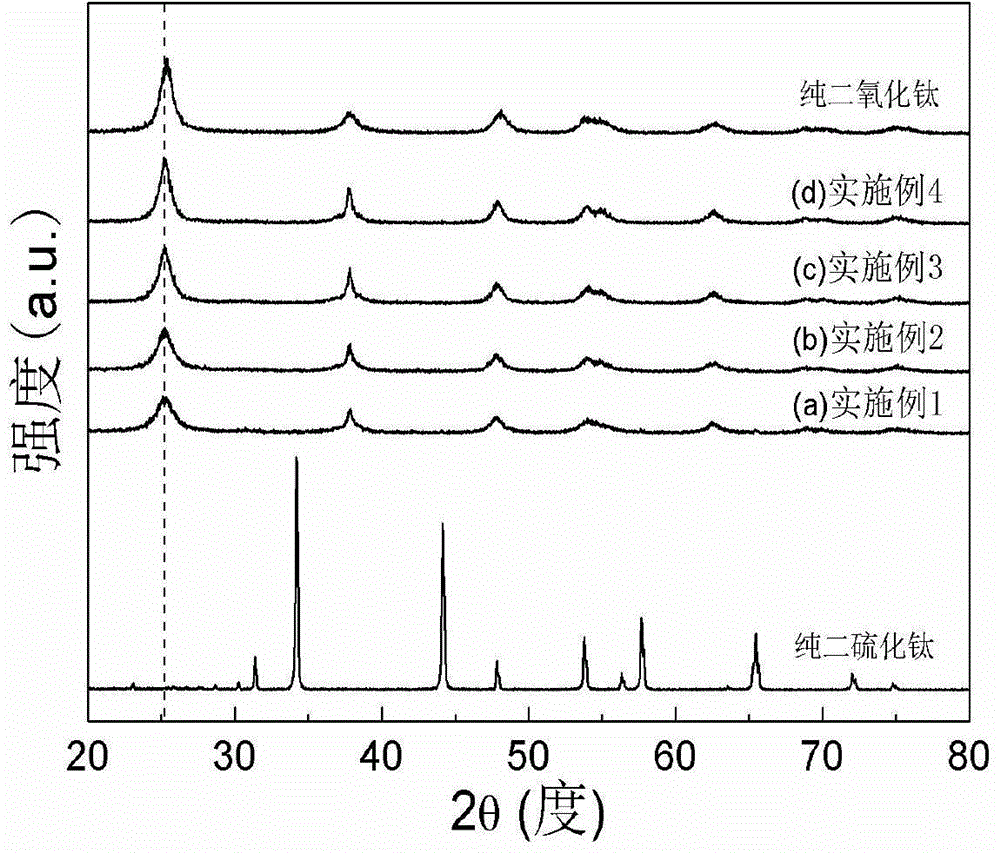
[0021] figure 1 It is the XRD figure of the sulfur-doped titanium dioxide photocatalyst that the present embodiment makes, and the dotted line in the figure corresponds to TiO 2 The main characteristic peak (101) diffraction peak, and pure TiS 2 and TiO 2 The comparison shows that the prepared nano-titanium dioxide photocatalyst is based on TiO 2 Anatase phase exists and there is no TiS in the product 2 components. TiO 2 (101) The diffraction peak shifts to a small angle...
Embodiment 2
[0023] Weigh 0.1 g TiS 2 The reaction raw materials were slowly added into 18 grams of deionized aqueous solution, fully stirred and mixed evenly. Then the mixed solution was put into a 20ml autoclave, sealed and placed in a muffle furnace, and reacted hydrothermally at 150° C. for 4 hours. Naturally cool to room temperature after the reaction, and dry at 80° C. after washing to obtain a light yellow sulfur self-doped titanium dioxide nanometer photocatalyst.
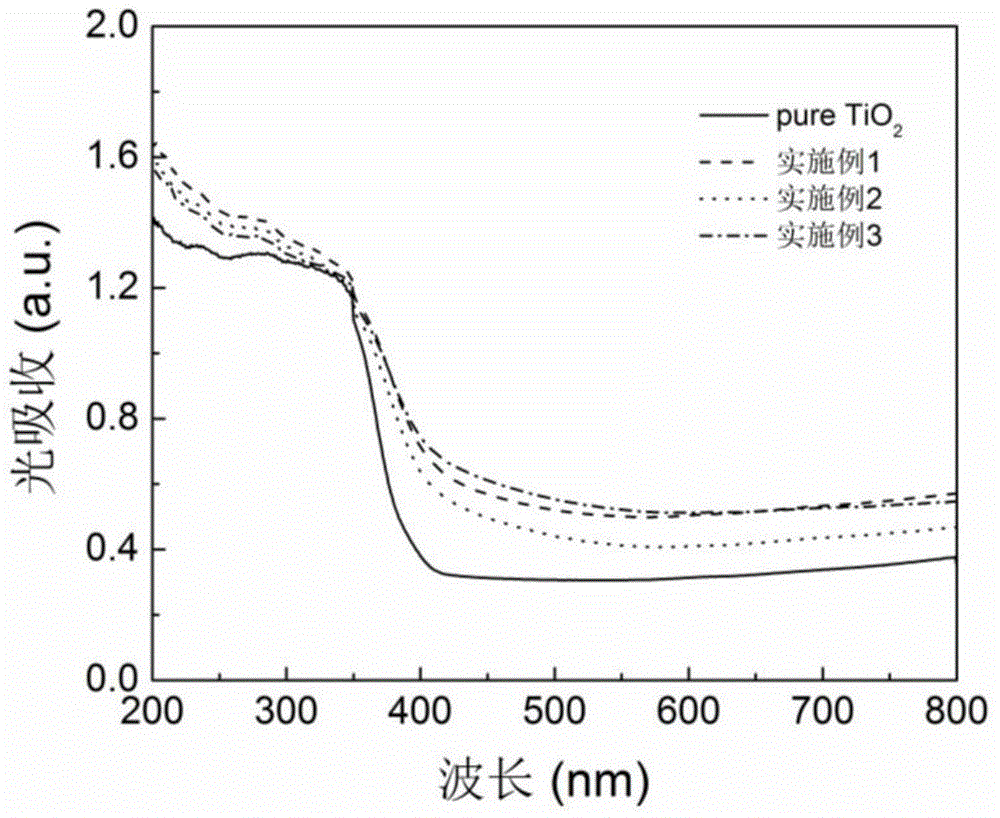
[0024] figure 1 The XRD pattern of the obtained sample is given, which is similar to that of pure TiS 2 and TiO 2 The comparison shows that the prepared nano titanium dioxide powder is based on TiO 2 Anatase phase exists and there is no TiS in the product 2 components. TiO 2 (101) The diffraction peak shifts to a small angle, proving that S atoms have replaced TiO 2 O-sites in the lattice. image 3 gives pure TiO 2 With the ultraviolet-visible absorption spectrogram of embodiment 2 product, prove that S self-d...
Embodiment 3
[0026] Weigh 0.1 g TiS 2 The reaction raw materials were slowly added into 18 grams of deionized aqueous solution, fully stirred and mixed evenly. Then the mixed solution was put into a 20ml autoclave, sealed and placed in a muffle furnace, and reacted hydrothermally at 180° C. for 4 hours. After the reaction is finished, it is naturally cooled to room temperature, washed and then dried to obtain a light yellow sulfur self-doped titanium dioxide nanometer photocatalyst.
[0027] figure 1 The XRD pattern of the obtained sample is given, which is similar to that of pure TiS 2 and TiO 2 The comparison shows that the prepared nano titanium dioxide powder is based on TiO 2 Anatase phase exists and there is no TiS in the product 2 components. TiO 2 (101) The diffraction peak shifts to a small angle, proving that S atoms have replaced TiO 2 O-sites in the lattice. image 3 gives pure TiO 2 With the ultraviolet-visible absorption spectrogram of embodiment 3 products, prove t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com