Nitro-containing curcumin derivative as well as preparation method and application thereof
A technology of curcumin derivatives and nitro groups, which is applied in the field of nitro-containing curcumin derivatives and their preparation, can solve the problems of low bioavailability, instability, poor water solubility, etc., and achieve low preparation cost and high structure Stable and achievable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
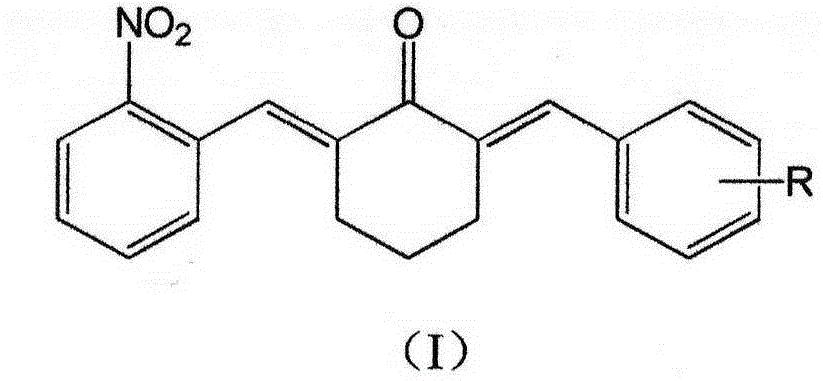
[0053] The invention provides a kind of preparation method of the curcumin derivative containing nitro, comprise making the substituent of the benzaldehyde with general formula (II) and formula (III) (E)-2-(2-nitrobenzylidene ) cyclohexanone undergoes aldol condensation reaction,
[0054]
[0055] Wherein R represents one or more substituents connected to the benzene ring, selected from H, halogen atoms, OH and optional lower alkoxy groups.
[0056] The preparation method of described nitro-containing curcumin derivatives specifically comprises the steps of: combining benzaldehyde substituents shown in formula (II) with formula (III) (E)-2-(2-nitrobenzylidene ) cyclohexanone is dissolved in the organic solvent a, catalyst b is added, and the reaction is complete at room temperature to obtain the reaction solution c, after the reaction solution c is post-treated and separated and purified, the curcumin derivative shown in the formula (I) is obtained; Described organic solve...
Embodiment 1
[0069] Preparation of (E)-2-(2-nitrobenzylidene)cyclohexanone
[0070] Cyclohexanone (9.8g, 0.1mol), morpholine (10.45g, 0.12mol) and p-toluenesulfonic acid (0.04g, 0.23mmol) were dissolved in 30mL of toluene, heated to reflux for 6h. After the reaction was cooled to room temperature, the toluene was evaporated off. Then, 4-(cyclohexanon-1-yl)morpholine (4.61g, 0.027mol) and o-nitrobenzaldehyde (4.0g, 0.023mol) were dissolved in 20mL of ethanol and reacted at 90°C for 6h. After the reaction was monitored by TLC, the pH was adjusted to 3 with 5% dilute hydrochloric acid. After the ethanol was evaporated by rotary evaporation, ethyl acetate was added for extraction, the organic layer was washed with water, washed with salt, and anhydrous MgSO 4 Dry, filter, and spin-dry the organic layer to obtain a crude product, and perform column purification to obtain a light yellow pure product with a yield of 51.7%.
Embodiment 2
[0072] Preparation of (2E, 6E)-2-(3,4-dimethoxybenzylidene)-6-(2-nitrobenzylidene)cyclohexanone (a9)
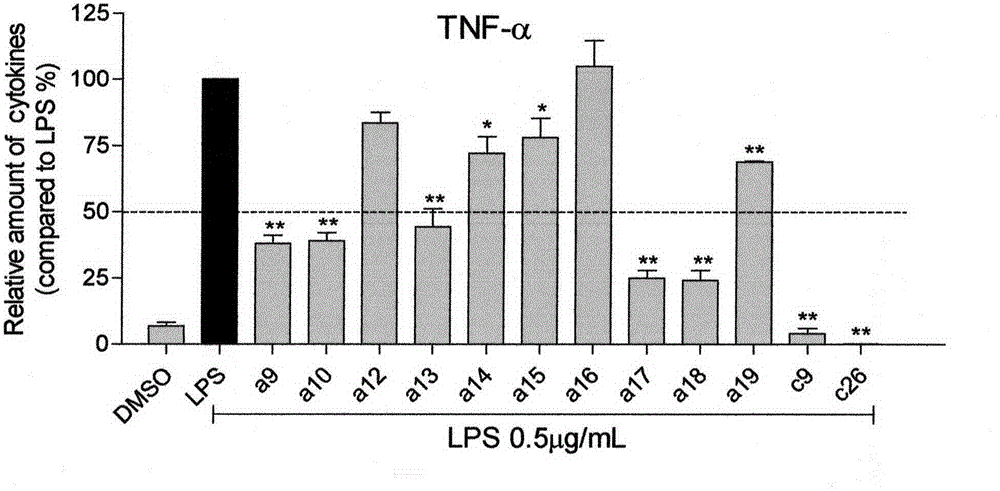
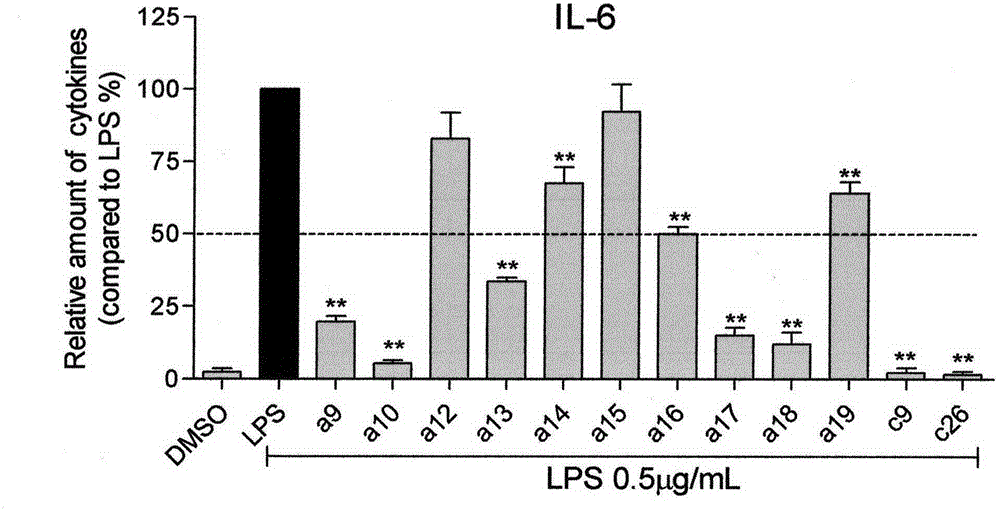
[0073] (E)-2-(2-nitrobenzylidene)cyclohexanone (100mg, 0.43mmol), 3,4-dimethoxybenzaldehyde (71.86mg, 0.43mmol) was dissolved in 6mL of absolute ethanol, Add 6 drops of 20% sodium hydroxide solution dropwise, react at room temperature for 24 hours, a yellow precipitate precipitates out of the solution, filter, wash with water and dilute ethanol until pure by TLC, and the yield is 70.6%.
[0074] Yellow powder, 70.6% yield, mp 131.8-135.5℃. 1 H NMR (600MHz, CDCl 3 ): δ (ppm) 8.225 (1H, d, J=7.2Hz, H-3), 7.947 (1H, s, H-β), 7.792 (1H, s, H-β'), 7.640 (1H, t , J=7.5Hz, H-4), 7.500(1H, t, J=7.8Hz, H-5), 7.370(1H, d, J=7.8Hz, H-6), 7.123(1H, d, J =8, 4Hz, H-6'), 7.027 (1H, s, H-2'), 6.915 (1H, d, J=8.4Hz, H-5'), 3.928 (3H, s, 3'-OCH 3 ), 3.914 (3H, s, 4'-OCH 3 ), 2.949 (2H, t, J=5.1Hz, Cyclohexanone-C H 2 CH 2 CH 2 -), 2.605 (2H, t, J=5.1Hz, Cyclohexanone-CH 2 CH 2 C H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com