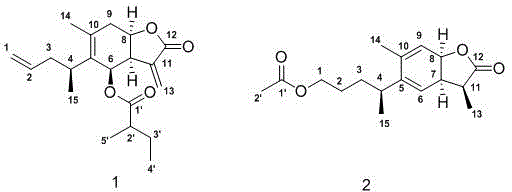
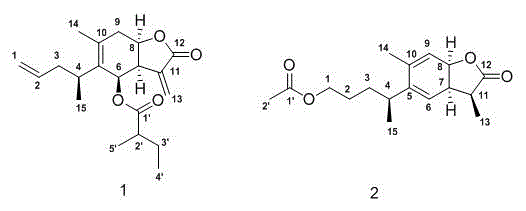
Sesquiterpene lactone compound, pharmaceutical composition comprising sesquiterpene lactone compound, as well as preparation method and application of sesquiterpene lactone compound
A technology of sesquiterpene lactone and compound, applied in the field of medicine, can solve problems such as no compound activity report, no literature report and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Inula multibranches (5kg) were crushed and soaked in 95% ethanol (3×15L) at room temperature for 24 hours, filtered, combined extracts, and recovered under reduced pressure to obtain a crude extract, suspended evenly with water, extracted with ethyl acetate 3 times to get ethyl acetate extract (385mg), the extract was decolorized by MCI and eluted with 95% ethanol, then used a silica gel column 10:0→1:1 chloroform-acetone gradient elution for crude fractionation, combined the same fractions , to get 6 parts F1-F6; F1 was purified by Sephadex LH-20 column chromatography chloroform:methanol=1:1 and then eluted by methanol-water gradient of RP-18 column chromatography 25:75→100:0 to obtain 5 small parts F1a -F1e, F1d was purified by silica gel column chloroform:ethyl acetate=5:1 and then eluted by HPLC methanol:water gradient 80:20 and 92:8 to obtain compounds 1 (21mg) and 2 (20mg);
[0022] Compound 1 structure confirmed: light yellow powder; HR-ESIMS showed [M+H] + It i...
Embodiment 2
[0029] Inula cotton wool (5kg) was crushed, soaked in 95% ethanol (3×15L) at room temperature for 24 hours, filtered, combined extracts, recovered ethanol under reduced pressure to obtain a crude extract, added water to suspend evenly, and extracted with ethyl acetate for 3 times, to obtain ethyl acetate extract (322mg), the extract was decolorized by MCI and eluted with 95% ethanol, then used a silica gel column 10:0 → 1:1 chloroform-acetone gradient elution for crude fractionation, and the same fractions were combined, 6 parts F1-F6 were obtained; F1 was purified by Sephadex LH-20 column chromatography chloroform:methanol=1:1 and then eluted by methanol-water gradient of RP-18 column chromatography 25:75→100:0 to obtain 5 small parts F1a- F1e and F1d were purified by silica gel column chloroform:ethyl acetate=5:1 and then eluted by HPLC methanol:water gradient 80:20 and 92:8 to obtain compounds 1 (17mg) and 2 (26mg).
[0030] See Example 1 for structure confirmation of compo...
Embodiment 3
[0032] Inula angustifolia (5kg) was crushed and soaked in 95% ethanol (3×15L) at room temperature for 24 hours, filtered, combined extracts, and recovered under reduced pressure to obtain a crude extract. Extracted 3 times to obtain ethyl acetate extract (350g), the extract was eluted with different concentrations of MCI methanol-water gradient, the elution gradient was 20%, 40%, 60%, 80%, 100%, and the same fractions were combined , get 5 parts F1-F5; Get F4 and obtain 4 small parts F4a-F4d through the chloroform-ethyl acetate gradient elution of silica gel column 9:1→3:2, get F4c through Sephadex LH-20 column chromatography chloroform:methanol= After 1:1 purification, compound 1 (19 mg) was semi-prepared by HPLC methanol:water=82:18; F5 was eluted with a gradient of petroleum ether-ethyl acetate on a silica gel column 9:1→2:3 to obtain 3 small parts of F5a -F5c, compound 2 (22 mg) was prepared by taking F5a and semi-preparing it by HPLC methanol:water=94:6;
[0033] The str...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com