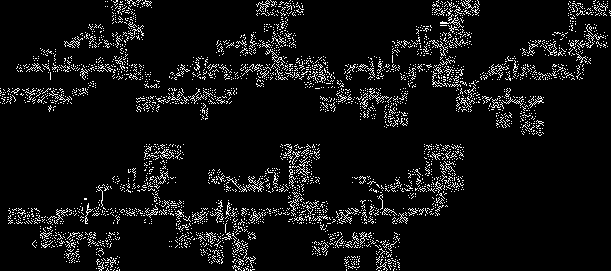
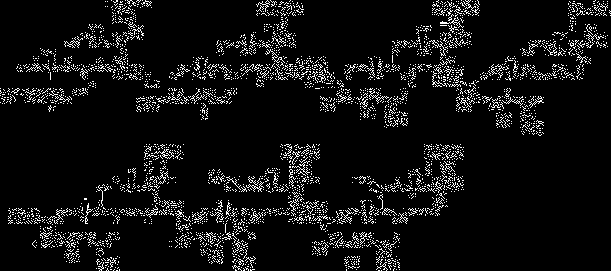
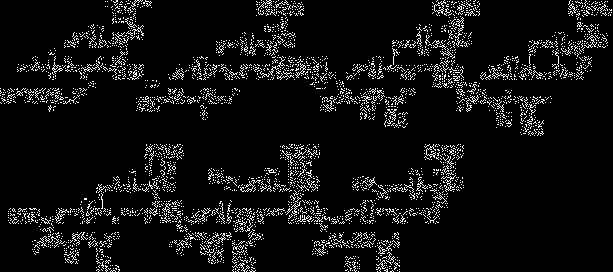Synthesis method of 6alpha-methylprednisolone
A technology of methylprednisolone and synthetic methods, applied in the direction of steroids, organic chemistry, fermentation, etc., can solve the problems of low reaction yield, difficult to eliminate selenium, large pollution, etc., achieve high yield, avoid dramatic The use of toxic chemicals, the effects of three wastes treatment and pressure relief
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0055] etherification reaction
[0056]Add 40ml of tetrahydrofuran into the four-neck flask, then add 10g of compound I, after nitrogen replacement, add 3ml of ethanol and 7ml of triethyl orthoformate; after raising the temperature to 36-38°C, add 0.2g of p-toluenesulfonic acid to continue the reaction 3 hours; TLC follow-up, cool down slightly to 30 after the reaction finishes + 1°C for use.
[0057] methylation reaction
[0058] Add 4gN-methylaniline and 37% formaldehyde 3g in the last step reactant; Slightly warm up to 40 + 1°C, keep warm for 3 hours; TLC detects the reaction status, after the reaction, the system cools down to about 15°C; concentrated hydrochloric acid adjusts the pH value of the system to 1-2; after the dropwise addition, the temperature rises to 25°C + 1°C, hydrolysis reaction for 2 hours; after adding 100ml of water dropwise into the reaction system, the system cooled down to 5 + After stirring at 1°C for 1 hour, after standing still for 5 hours, su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com