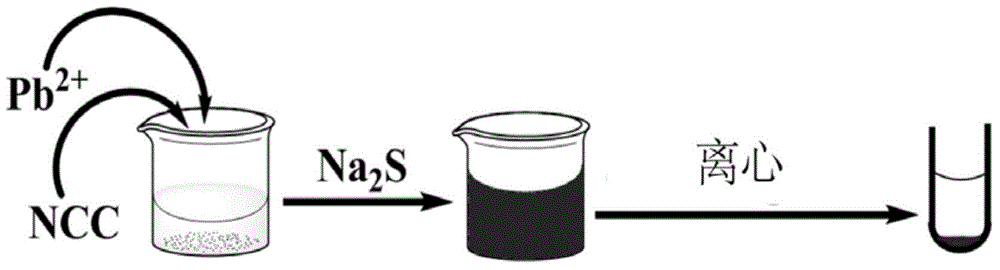
Lead ion detecting and removing method based on nanocrystalline cellulose
A nanocrystalline cellulose, lead ion technology, applied in the measurement of color/spectral properties, material analysis by observing the effect on chemical indicators, and analysis by chemical reaction of materials, etc., can solve the problem that lead ions cannot be removed, The operation steps are complicated, and the color developer is required to achieve the effect of easy popularization and application, simple operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the influence of NCC concentration on detection result
[0038] (1) Prepare 2.5mg / mL, 5mg / mL, 10mg / mL nanocrystalline cellulose solutions and 16% acetic acid solution, take different concentrations of nanocrystalline cellulose solution 0.12mL, acetic acid solution 0.15mL and 0.21mL respectively ionized water mix;
[0039] (2) Add 0.06mL of lead ion solution to be tested in step (1) solution, mix well;
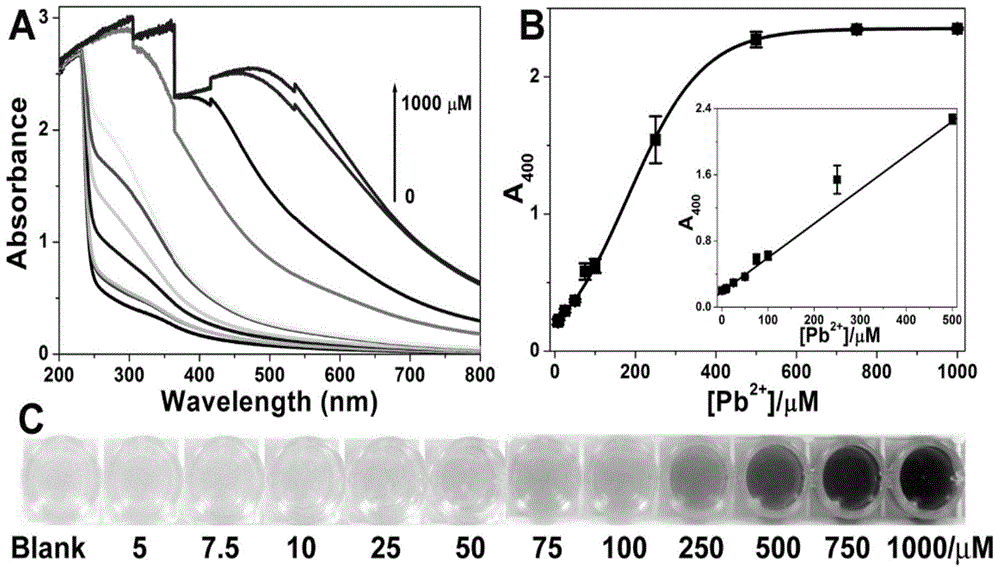
[0040] (3) Add 0.06 mL of 10 mM sodium sulfide solution to the uniformly mixed solution in step (2), react for 10 minutes, observe the color change of the solution, and then detect the absorbance at 400 nm with a UV-Vis spectrophotometer.
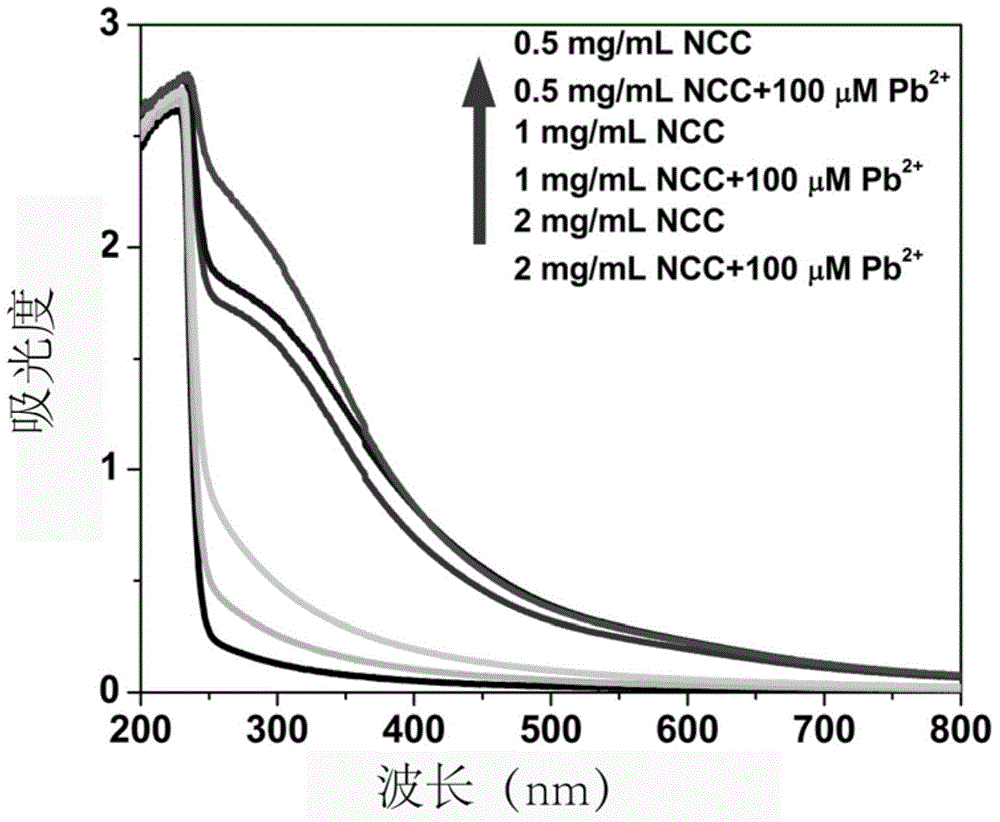
[0041] from figure 2It can be seen that when the concentration of NCC is low, it is not easy to disperse lead sulfide. When the concentration of NCC is high, its solubility is poor, and it is easy to produce flocculent precipitation when adding a solution containing lead ions. And observe the change of the absorption...
Embodiment 2
[0042] Embodiment 2: the influence of sodium sulfide concentration on detection result
[0043] (1) Prepare 5mg / mL nanocrystalline cellulose solution and 16% acetic acid solution, mix 0.12mL nanocrystalline cellulose solution, 0.15mL acetic acid solution and 0.21mL deionized water;
[0044] (2) Add 0.06mL of lead ion solution to be tested in step (1) solution, mix well;
[0045] (3) Prepare sodium sulfide solutions at concentrations of 5mM, 10mM, 20mM, 40mM, 60mM, 80mM and 100mM, and use them first.
[0046] (4) Then add 0.06 mL of sodium sulfide solutions of different concentrations to the uniformly mixed solution in step (2), and the final concentrations of sodium sulfide are 0.5 mM, 1 mM, 2 mM, 4 mM, 6 mM, 8 mM and 10 mM. React for 10 minutes, observe the color change of the solution, and then detect the absorbance at 400 nm with an ultraviolet-visible spectrophotometer.
[0047] The concentration and time of sodium sulfide have no significant effect on the detection resu...
Embodiment 3
[0048] Embodiment 3: the influence of reaction time on detection result
[0049] (1) Prepare 5mg / mL nanocrystalline cellulose solution and 16% acetic acid solution, mix 0.12mL nanocrystalline cellulose solution, 0.15mL acetic acid solution and 0.21mL deionized water;
[0050] (2) Add 0.06mL of lead ion solution to be tested in step (1) solution, mix well;
[0051] (3) Add 0.06mL of 10mM sodium sulfide solution to the uniformly mixed solution in step (2), react respectively for 3 minutes, 5 minutes, 10 minutes, 20 minutes, 30 minutes, 40 minutes, 50 minutes and 60 minutes, observe The color of the solution changes, and the absorbance value at 400 nm is detected with a UV-visible spectrophotometer.
[0052] The experimental results show that the reaction is complete when the reaction time is 10 minutes, and the reaction time is 10 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com