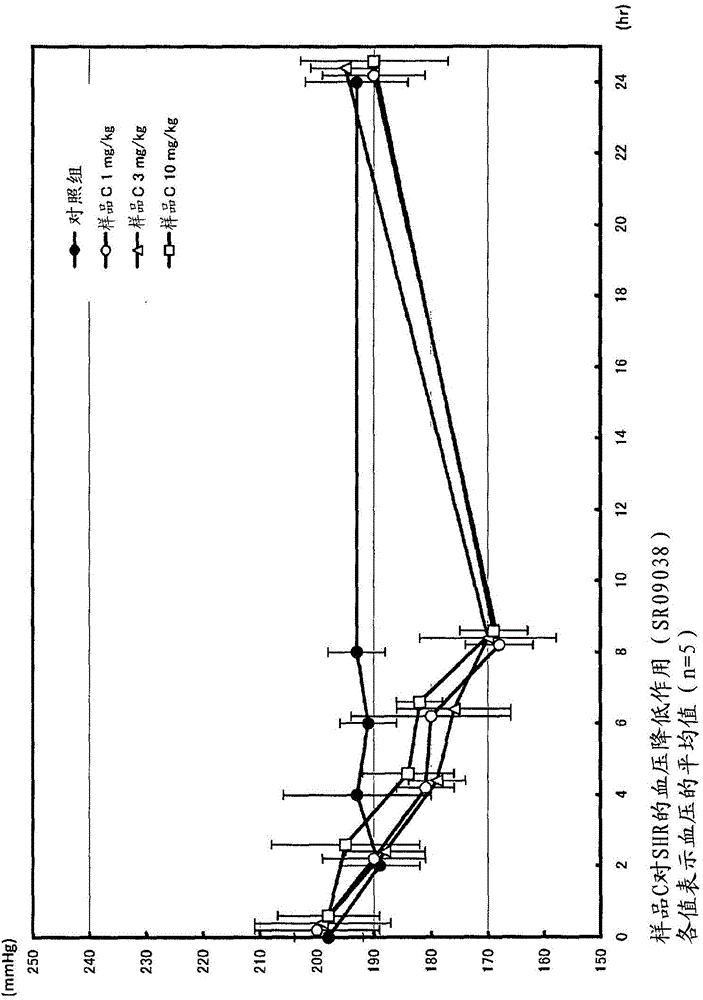
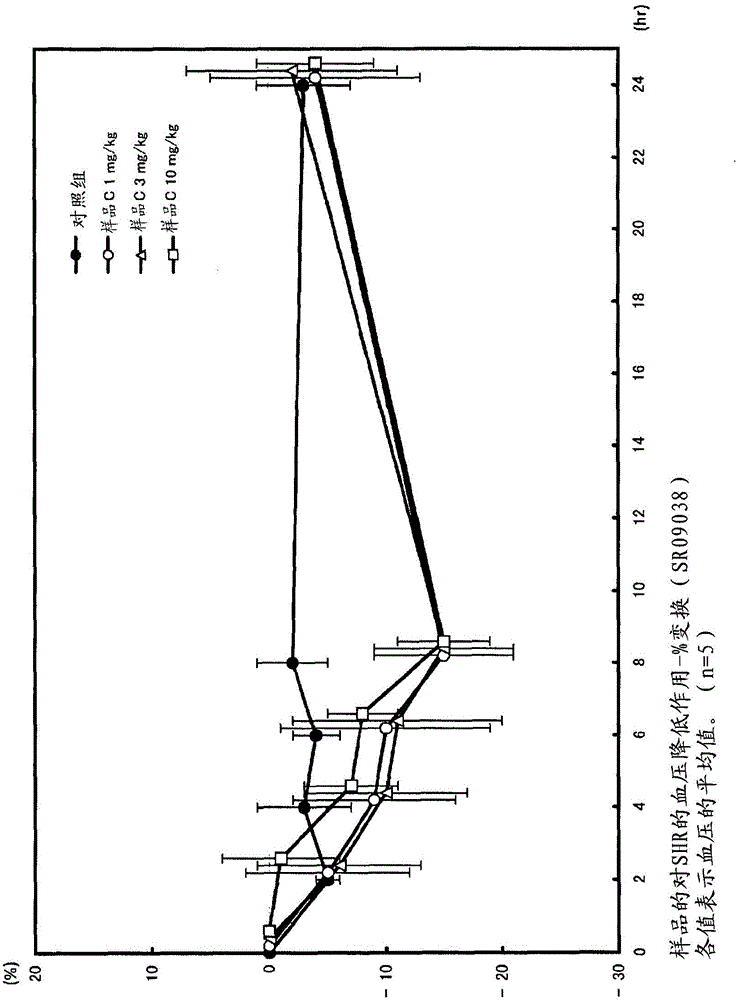
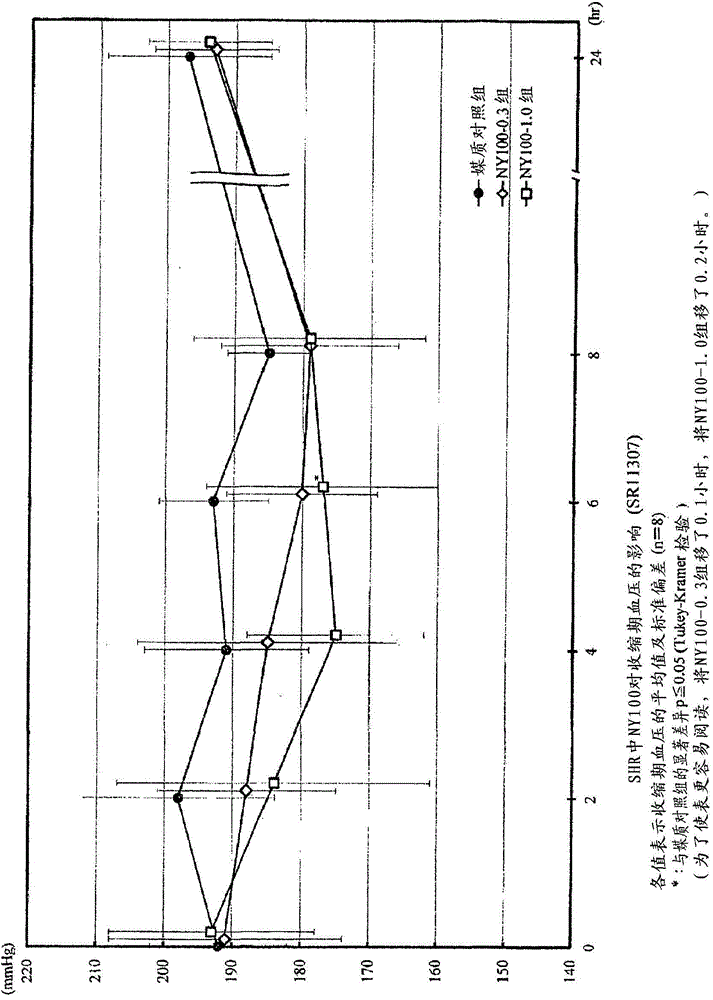
Angiotensin-converting-enzyme inhibiting dipeptide
A sequence, tryptophan technology, applied in the field of 15 kinds of dipeptides and peptide compositions containing them, can solve problems and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] [(a) Industrial production of peptide composition having ACE inhibitory activity (1)]
[0109] Add 10,000 L of water to 1,000 kg of bonito knuckle protein, heat treatment (95°C, 35 minutes), remove amino acids and water-soluble proteins, add 2,884 L of water to the obtained hot water extraction residue 1,153 kg (576 kg of protein), and adjust the pH to 7 with 6N caustic soda. Protein NY100 (Amano enzyme) enzyme 2.0wt% (per protein enzyme amount) was added, and it was made to react at 50 degreeC for 20 hours, stirring. After the reaction, add caustic soda to adjust the pH to 6.8, and heat at 98°C for 15 minutes to inactivate the enzyme. Thereafter, the undecomposed protein was removed by a vibration filter, a Delaval centrifuge, and a centrifuge, and the supernatant was filtered and clarified with celite to obtain a decomposed liquid (200 kg of protein). ACE inhibitory activity IC 50 The value is 0.136 mg / ml (protein).
[0110] 200 kg of this peptide was loaded to the...
Embodiment 2
[0199] [(a) Industrial production of peptide composition having ACE inhibitory activity]
[0200] Add 10,000L of water to 1,000kg of skipjack protein, heat treatment (95°C, 35 minutes), remove amino acids and water-soluble proteins, add 4,436L of water to the obtained hot water extraction residue 1,774kg (886kg of protein), and adjust the pH to 7 with 6N caustic soda 1.0 wt% (per protein enzyme amount) of THERMOASE PC10F (Yamato Kasei) enzyme was added and reacted at 50° C. for 17 hours while stirring. After the reaction, add caustic soda to adjust the pH to 6.8, and heat at 98°C for 15 minutes to inactivate the enzyme. Thereafter, the undecomposed protein was removed by a vibrating filter, a Delaval centrifuge, and a centrifuge, and the supernatant was filtered with diatomaceous earth to obtain an enzymatic decomposition solution (200 kg of protein). ACE inhibitory activity IC 50 The value is 0.204 mg / ml (protein).
[0201] 200 kg of this peptide was loaded to the hydropho...
Embodiment 3
[0260] Synthesis of Peptides by Synthesis:
[0261] Using the automatic peptide synthesizer (ABI 430 model) of Applied Biosystems, according to the program, the peptide chain was extended from the C-terminus by the BOC method to synthesize the target protected peptide resin.
[0262] After the construction of the peptide on the resin is completed, the peptide resin is dried to protect it. Deprotection of the resulting protected peptide and cleavage of the peptide from the resin support were performed by anhydrous hydrogen fluoride treatment (HF / p-Creso18: 2 v / v, 60 minutes). The resulting crude peptide was extracted with 90% acetic acid and obtained as a powdered solid by lyophilization. Furthermore, the obtained crude peptide was loaded on a high performance liquid chromatography using an ODS column and purified to obtain the target peptide.
[0263] Column: YMC-Pack ODS-A (30mmID×250mmL, YMC)
[0264] Mobile layer: Buffer A: 5% CH 3 CN, 0.1% TFA
[0265] Buffer B: 40% C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com