Ivabradine hydrochloride single-layer osmotic pump controlled release tablet and preparation method thereof
An ivabradine hydrochloride, single-layer osmotic pump technology, applied in osmotic delivery, coating, pharmaceutical formulations, etc., can solve the problems of high requirements for equipment, complicated preparation process, slow release rate of sustained-release matrix tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
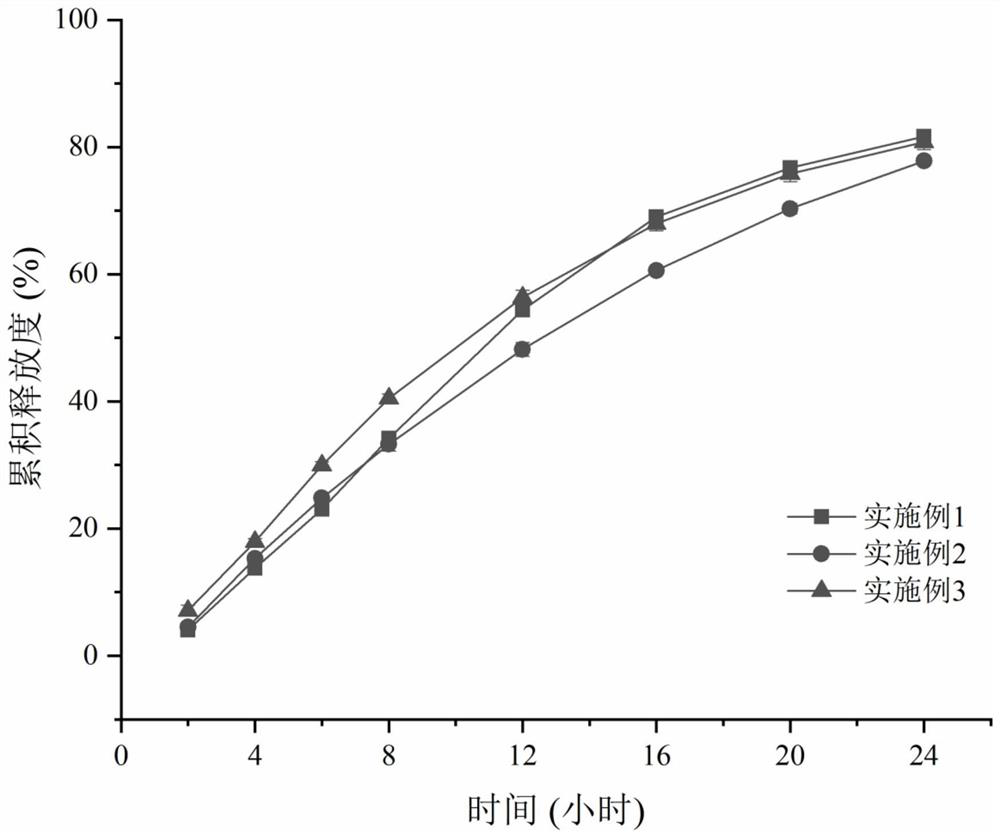
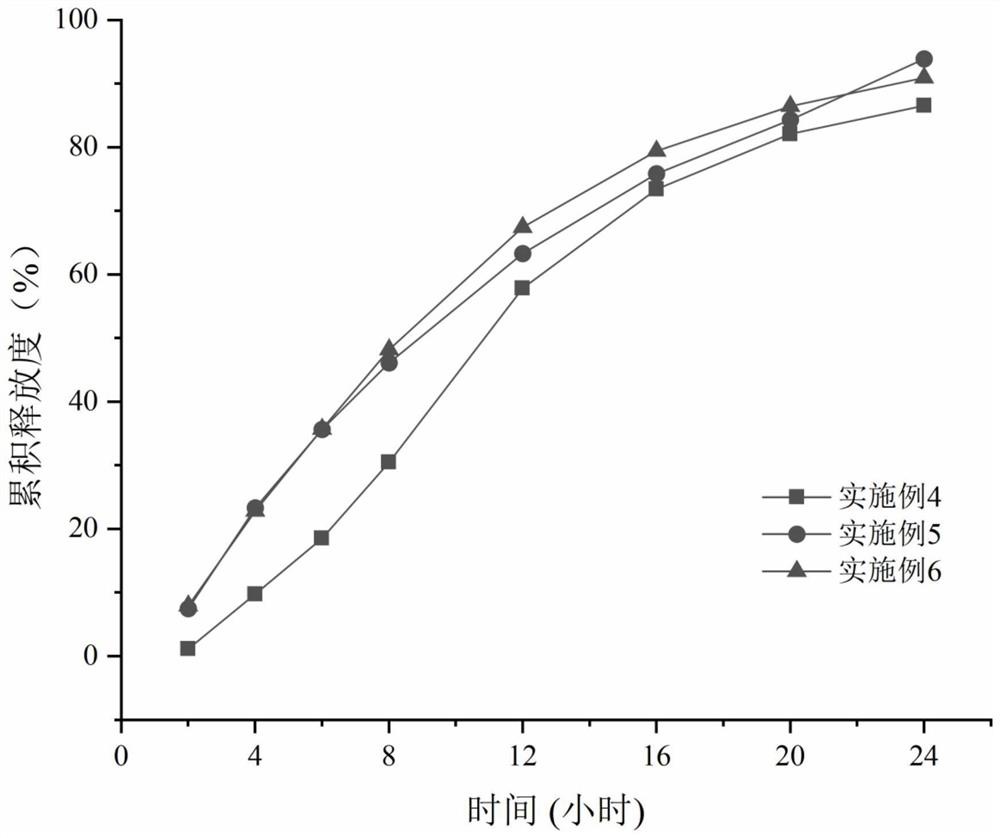
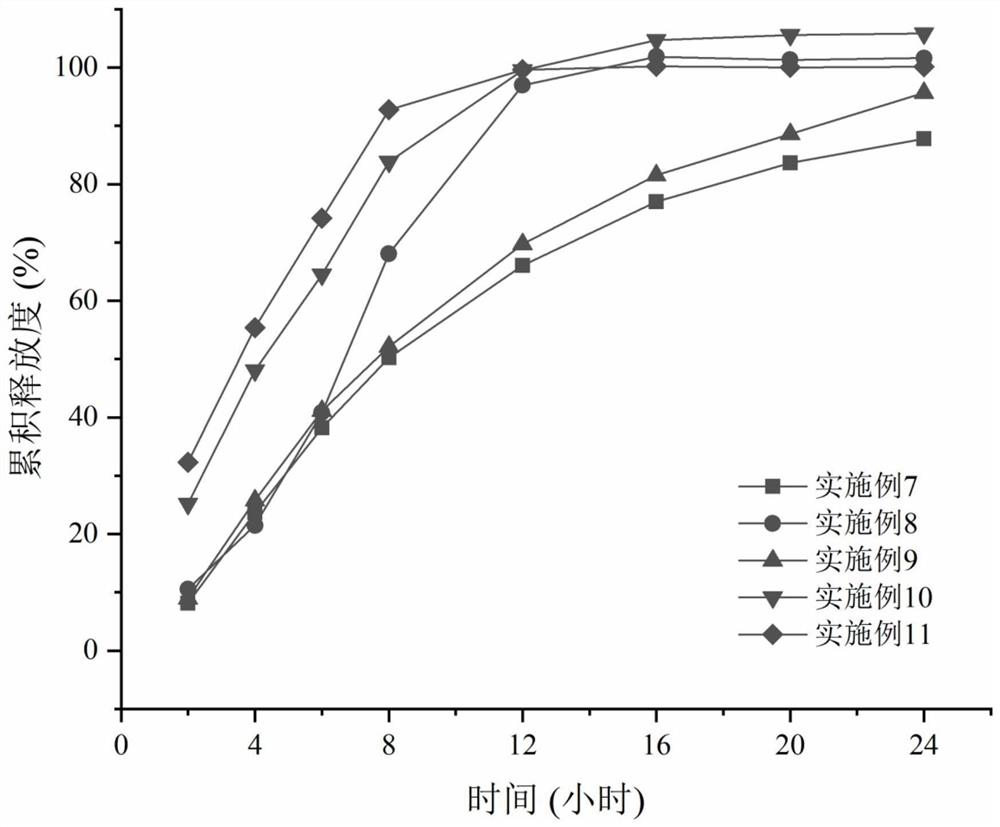
Examples
Embodiment 1~ Embodiment 11
[0025] Example 1-Example 11 Preparation method: Take the prescribed amount of medicine and auxiliary materials (except magnesium stearate), pass through a 30-mesh sieve and mix evenly. Add an appropriate amount of wetting agent absolute ethanol to granulate, pass through a 20-mesh sieve for wet granulation, dry in an oven at 40°C for 2 hours, pass through a 20-mesh sieve to dry and granulate. Add magnesium stearate and mix. Circular dimpled film-pressed tablets with a diameter of 8mm and a hardness of 5-9kg. Weigh cellulose acetate and polyethylene glycol 3350 according to the prescription, heat in a constant temperature water bath at 35°C, and add them into acetone-water solution (99.5:0.5) under magnetic stirring. After completely dissolving, the coating solution is clear and transparent. The tablet core is placed in the coating pan, the temperature of the tablet bed is controlled at 22-28° C., and the weight gain of the coating is 5-6%. After the coating is completed, pla...
Embodiment 1
[0028] Tablet core prescription
[0029] composition Dosage (per tablet) Ivabradine Hydrochloride 10.8mg Hydroxyethyl Cellulose LR 30mg Hypromellose E5 10mg Mannitol 147.2mg Magnesium stearate 2mg
[0030] Coating prescription
[0031] composition Dosage (per 100 tablets) Cellulose acetate 0.9g polyethylene glycol 3350 0.1g acetone 19.9g water 0.1g
Embodiment 2
[0033] Tablet core prescription
[0034] composition Dosage (per tablet) Ivabradine Hydrochloride 10.8mg Hypromellose E50 30mg Hypromellose E5 10mg Mannitol 147.2mg Magnesium stearate 2mg
[0035] Coating prescription
[0036] composition Dosage (per 100 tablets) Cellulose acetate 0.9g polyethylene glycol 3350 0.1g acetone 19.9g water 0.1g
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com