Nonaqueous electrolyte solution and nonaqueous electrolyte secondary battery using same
A non-aqueous electrolyte and secondary battery technology, which is applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolyte battery electrodes, secondary batteries, etc., can solve the problems of battery capacity reduction, battery material degradation, safety reduction, etc. The effect of excellent gas generation and cycle characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A-1
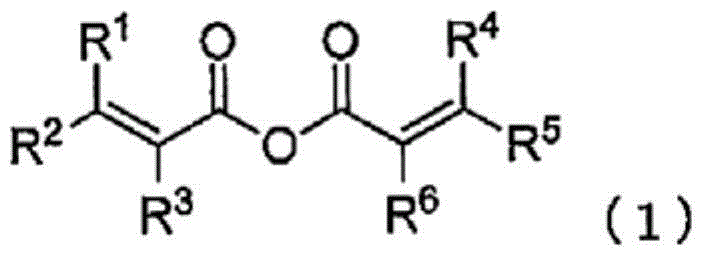
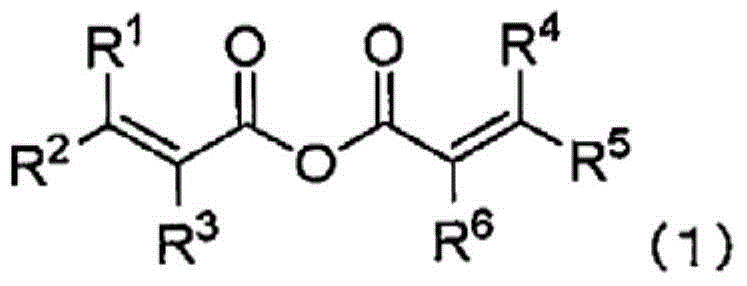
[0470] Under a dry argon atmosphere, ethylene carbonate (EC) as a cyclic carbonate, dimethyl carbonate (DMC) as a chain carbonate, and ethyl methyl carbonate (EMC) as EC:DMC:EMC=30 : 30:40 volume ratio, add fully dried LiPF so that it becomes 1.0 mol / L 6 (The resulting mixture is referred to as "reference electrolyte"). With respect to the whole reference electrolytic solution, methacrylic anhydride was added so that it might become 0.5 mass %, and vinylene carbonate (VC) would be added so that it might become 1 mass %, and the non-aqueous electrolytic solution was prepared.
Embodiment 1A-2~1A-8
[0471] [Examples 1A-2 to 1A-8, Comparative Examples 1A-1 to 1A-8, Reference Examples 1A-1 to 1A-11]
[0472] A reference electrolyte solution was prepared in the same manner as in Example 1A-1, and the compounds listed in Table 1 below were added in proportion to the entire obtained reference electrolyte solution to prepare each non-aqueous electrolyte solution. Among them, Comparative Example 1A-1 is the reference electrolyte solution itself.
[0473]
[0474] In 98 parts by mass of graphite powder as the negative active material, add 1 part by mass of the aqueous dispersion of carboxymethylcellulose sodium and 1 part by mass of the aqueous dispersion of styrene-butadiene rubber as thickener and binding agent , mixed with a disperser to make a slurry. The obtained slurry was applied to one surface of a copper foil, dried, and pressurized, and the negative electrode was cut into circular shapes with a diameter of 12.5 mm and used. The prepared negative electrode was used a...
Embodiment 2A-1~2A-5、 comparative example 2A-1~2A-7
[0491] [Examples 2A-1 to 2A-5, Comparative Examples 2A-1 to 2A-7, Reference Examples 2A-1 to 2A-6]
[0492] A reference electrolyte solution was prepared in the same manner as in Example 1A-1, and the compounds described in the following Table 2 were added in proportion to the entire obtained reference electrolyte solution to prepare each non-aqueous electrolyte solution, and a coin-type battery was produced. Table 2 shows the results of the initial charge-discharge efficiency (%) obtained by (initial discharge capacity / initial charge capacity)×100. In addition, the examples, comparative examples, and reference examples described in Table 2 all used the same reference electrolyte solution as in Table 1.
[0493] [Table 2]
[0494] Table 2
[0495]
[0496] In Comparative Example 2A-2 using methacrylic anhydride, which is a compound represented by formula (1), the initial charge-discharge efficiency decreased compared with Comparative Example 2A-1 using only the standard ele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com