Halloysite nanotube drug sustained-release material and preparation method thereof
A technology of halloysite nanotubes and slow-release materials, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. It has universality and other issues, and achieves the effects of improving biological activity, increasing the probability of complications, and diversifying the way of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
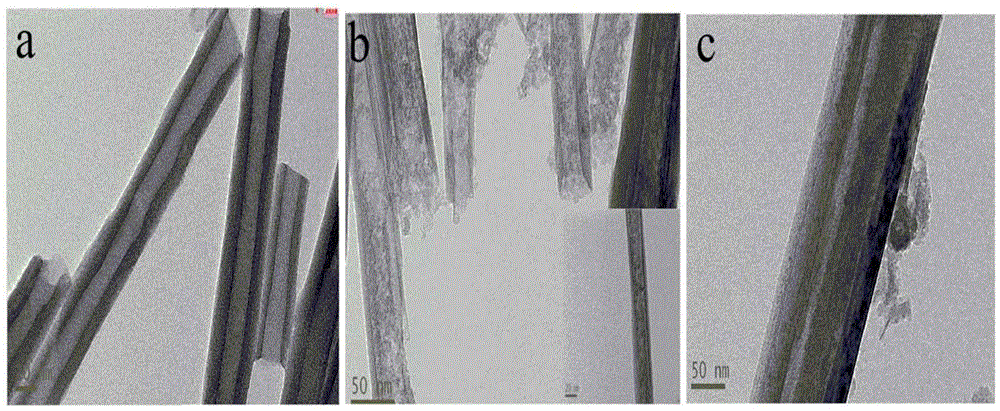
[0040] (1) Corrosion of halloysite nanotubes (HNTs)
[0041] Take 4g of halloysite powder and disperse it in 200mL of 3mol / L dilute sulfuric acid solution. After ultrasonic dispersion, stir magnetically in a water bath at 70°C for 12h, wash with a large amount of distilled water until neutral, and dry at 60°C in vacuum to obtain the partially corroded expansion of the inner wall. Halloysite nanotubes.
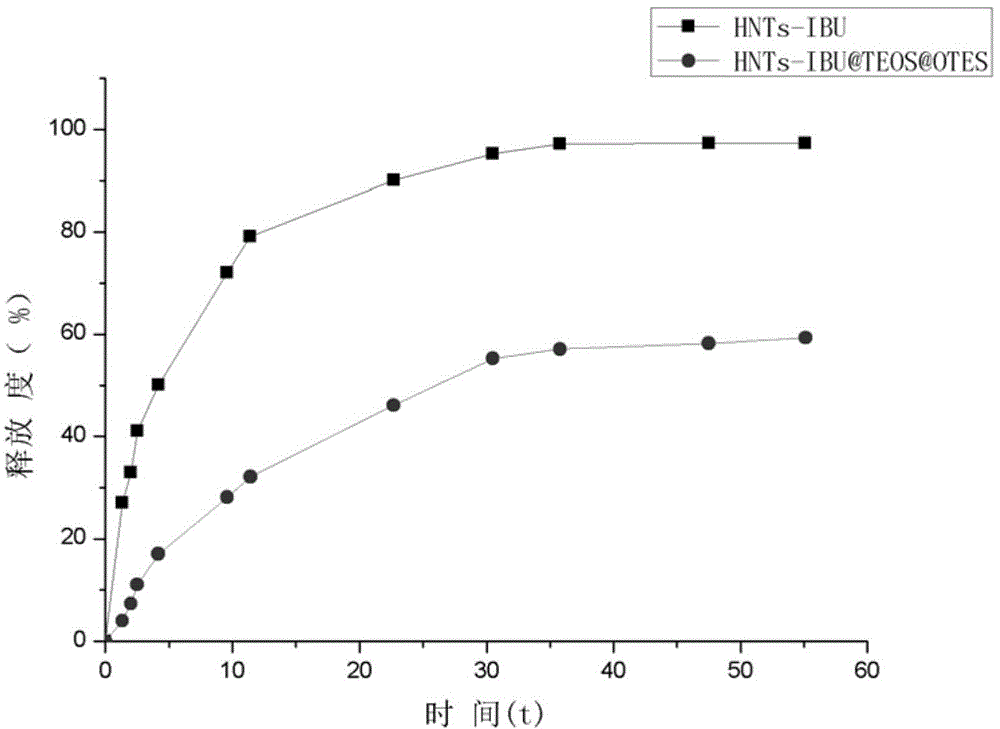
[0042] (2) Load of ibuprofen (IBU)
[0043] Take 1g of ibuprofen sample in a conical flask, dissolve it completely with 40mL of ethanol, then add 1g of expanded halloysite nanotubes for ultrasonic dispersion, and ensure that the system is in a vacuum environment, and shake at constant temperature for 24 hours to achieve drug loading. For the purpose, wash completely with ethanol and water, and vacuum-dry to obtain HNTs-IBU.
[0044] (3) Hydrophobic layer modification
[0045]a. Dissolve 0.5g of HNTs-IBU with 200mL of solvent (ethanol: water = 4:1) by ultrasound, stir magneti...
Embodiment 2
[0060] (1) Corrosion of halloysite nanotubes
[0061] Take 4g of halloysite powder and disperse it in 200mL of 5mol / L dilute sulfuric acid solution. After ultrasonic dispersion, stir magnetically in a water bath at 80°C for 12h, wash with a large amount of distilled water until neutral, and dry at 70°C in vacuum to obtain the partially corroded expansion of the inner wall. Halloysite nanotubes.
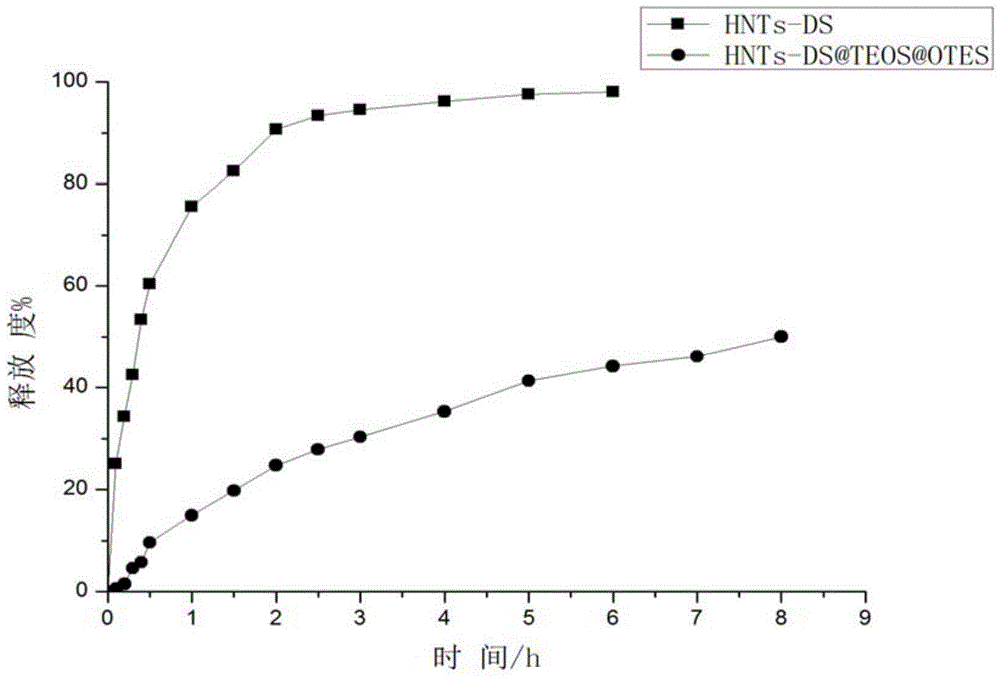
[0062] (2) Load of ofloxacin (OFL)
[0063] Take 1g ofloxacin sample in a conical flask, dissolve it completely with 30mL of dilute acetic acid solution with pH 3.0, then add 2g of expanded halloysite nanotubes for ultrasonic dispersion, and ensure that the system is in a vacuum environment and shake at constant temperature After 48 hours to achieve the purpose of drug loading, it was washed completely with dilute acetic acid solution and dried in vacuum to obtain HNTs-OFL.
[0064] (3) Hydrophobic layer modification
[0065] a. 0.5g of HNTs-OFL was ultrasonically dissolved with 20...
Embodiment 3
[0070] (1) Corrosion of halloysite nanotubes
[0071] Take 4g of halloysite powder and disperse it in 200mL of 4mol / L dilute sulfuric acid solution. After ultrasonic dispersion, stir magnetically in a water bath at 80°C for 12h, wash with a large amount of distilled water until neutral, and dry at 70°C in vacuum to obtain the partially corroded expansion of the inner wall. Halloysite nanotubes.
[0072] (2) Load of ibuprofen (IBU)
[0073] Take 2g of ibuprofen sample in a conical flask, dissolve it completely with 50mL of ethanol, then add 2g of expanded halloysite nanotubes for ultrasonic dispersion, and ensure that the system is in a vacuum environment, and shake at constant temperature for 24 hours to achieve drug loading. For the purpose, wash completely with ethanol and water, and vacuum-dry to obtain HNTs-IBU.
[0074] (3) Hydrophobic layer modification
[0075] a. Dissolve 0.5g of HNTs-IBU with 200mL of solvent (ethanol: water = 5:1) by ultrasound, stir magnetically ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com