Preparation method of carbon-base adsorbing material capable of selectively adsorbing copper ions
An adsorption material and selective technology, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the problem that the adsorption material selective adsorption coefficient is not ideal, harmful initiators and surfactants, toxic problem, to achieve the effect of high selectivity, high adsorption selectivity, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of carbon-based adsorbent materials with selective adsorption for copper ions.
[0023] Mix tetraethylenepentamine (TEPA), cupric chloride, and glucose according to the molar ratio of 1:1:3~1:1:10 and stir evenly, then put them into the reaction kettle, and react at 180°C for 15 hours, and the obtained solids are used 0.01mol / L EDTA solution was repeatedly soaked and washed, and finally washed with absolute ethanol until the filtrate was colorless and transparent, and the washed solid was dried at 80°C to obtain the carbon-based adsorption material containing amino groups.
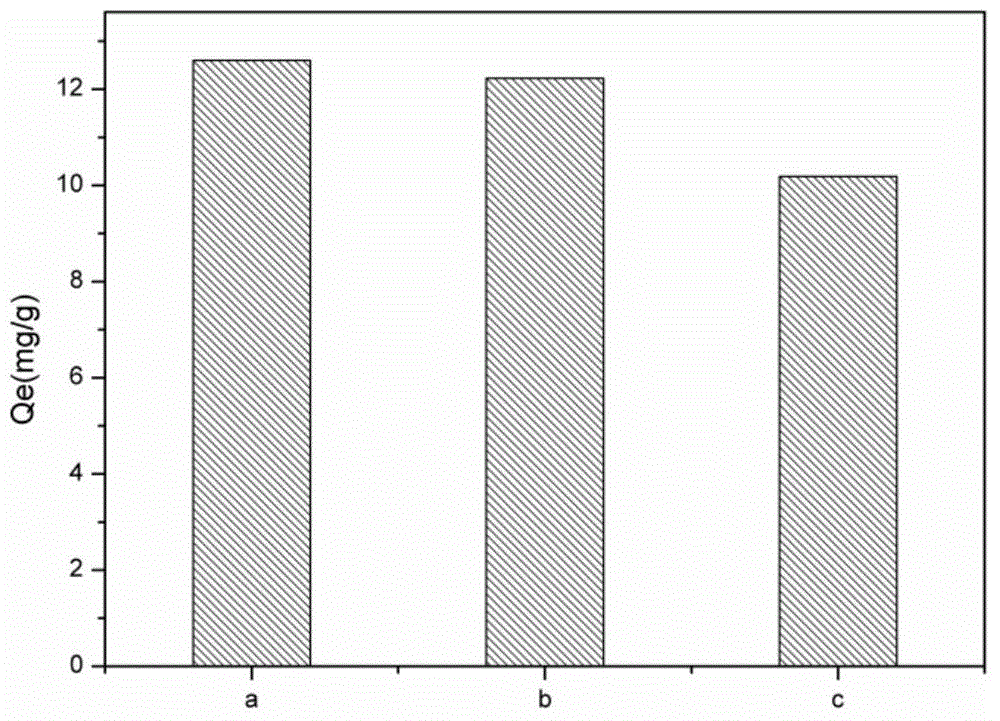
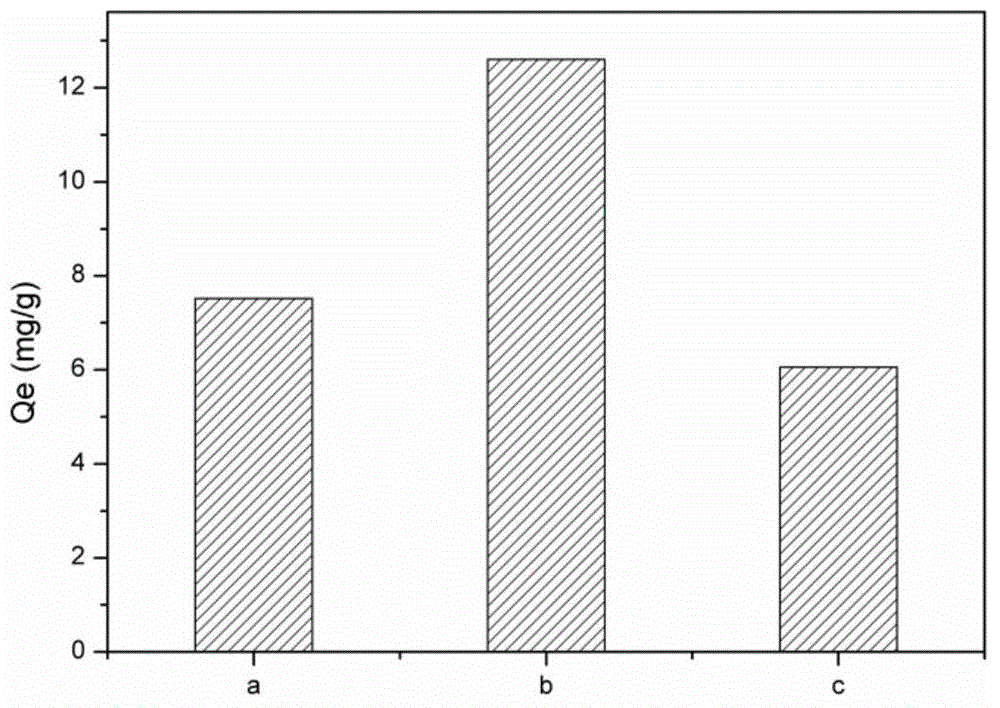
[0024] Such as figure 1 As shown, when TEPA, copper ions, and glucose were mixed at a molar ratio of 1:1:5, the carbon-based adsorbent material had the largest adsorption capacity for copper ions. Compared with non-ion imprinted adsorption materials and commercial activated carbon prepared under the same conditions, such as figure 2 As shown, the copper ion imprinted adsorption materi...
Embodiment 2
[0026] Selective adsorption experiments of carbon-based adsorbent materials prepared by copper ion imprinting.
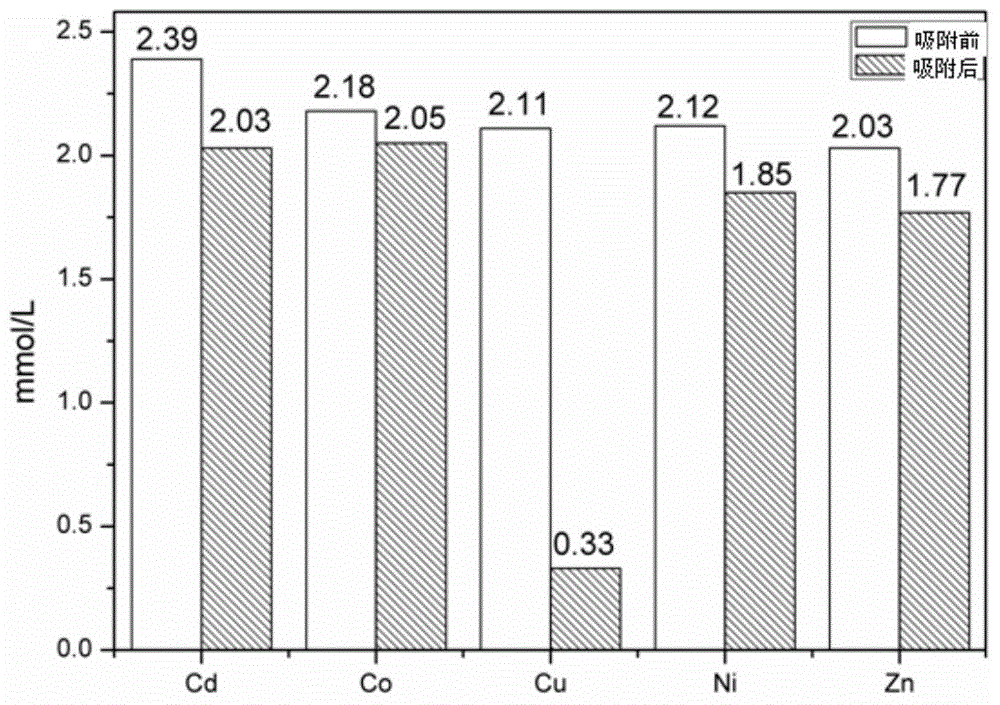
[0027] In a 25-mL centrifuge tube, add 10 mL containing a certain concentration of Cu 2+ 、Cd 2+ 、Co 2+ 、Ni 2+ , Zn 2+ Ionic solutions (Cd(II), Co(II), Cu(II), Ni(II), Zn(II), 0.002mol / L), the exact concentrations are 2.39mmol, 2.18mmol, 2.11mmol, 2.12mmol , 2.03mmol, and then add 100mg of copper ion imprinted adsorption material, shake well, ultrasonic for 30min, stand for adsorption for 24h, and then detect the supernatant by ICP.
[0028] Experimental results such as image 3 As shown, the adsorption rate of the adsorption material prepared by the copper ion imprinting method for copper ions reached 84.4%, while the adsorption rates for the other four ions were all very small. 2+ to Cd 2+ 、Co 2+ 、Ni 2+ , Zn 2+ The ion-selective adsorption coefficients are 31.8, 90.2, 38.6, and 36.1, respectively, and the copper ion imprinted adsorption material has very ...
Embodiment 3
[0030] Effect of pH value on the adsorption capacity of copper ion imprinted template adsorption materials.
[0031] Use 0.1 mol / L hydrochloric acid solution to adjust the pH to 1-5, add 50 mg of copper ion imprinted adsorption material to 5 mL of copper ion solution with an initial concentration of (800 mg / L), ultrasonicate for 30 min, and let stand for adsorption for 24 h. After centrifugation, the supernatant was taken to measure the copper ion concentration Ce of the adsorbed solution by DDTC chromogenic method. Calculate the adsorption amount Q (mg / g) and the adsorption rate E according to the following formula.
[0032] Q=(C 0 -C e )V / m
[0033] E=(C 0 -C e ) / C0 *100%
[0034] where C 0 is the initial concentration of copper ions (g / L), C e is the adsorption equilibrium concentration of copper ion (g / L), V is the volume of the adsorption system (mL), and m is the mass of the adsorbent (g). When the amount of adsorption material is 10g / L and the concentration of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com