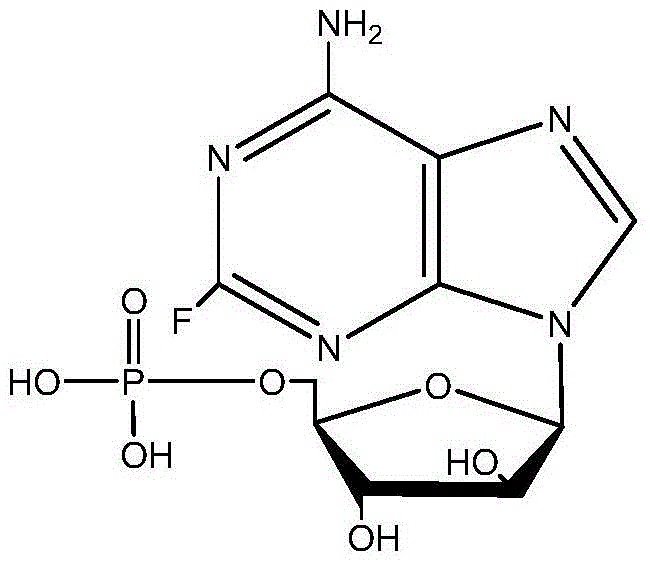
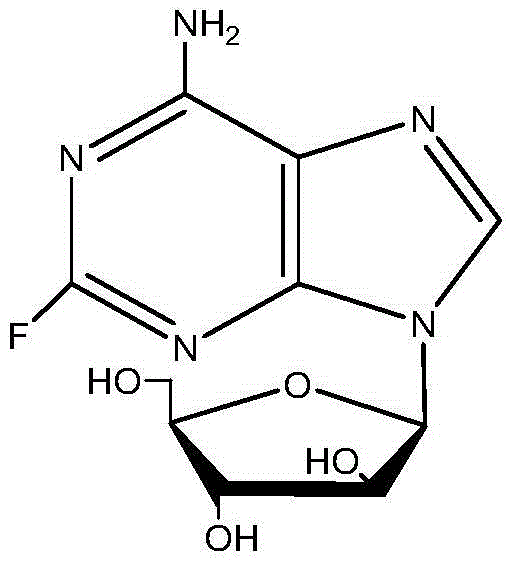
Preparation method for 9-beta-D-arabinofuranosyl-2-fluoroadenine-5'-phosphate
A technology of triethyl phosphate and fludarabine phosphate, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of complicated post-processing operations, harsh reaction temperatures, and cumbersome refining steps. Achieve the effects of simple and easy operation, short reaction time and high purity after reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under stirring conditions, put fludarabine (10g), triethyl phosphate (115mL), and phosphorus oxychloride (7.5mL) in a low-temperature circulation pump at -5 to -10°C, while maintaining the internal temperature at -5 ~-1°C; Stir the reaction at -5~-1°C for 15 hours; when the amount of fludarabine is less than 2% in the HPLC detection area, the reaction is considered complete, and then pure water (105mL) is added under stirring The obtained mixture was washed with dichloromethane, the obtained aqueous phase was adjusted to pH = 3 with 50% NaOH solution, concentrated under reduced pressure at 35-40°C, refrigerated for crystallization, filtered, and the filter cake was washed with a small amount of ethanol and water to obtain a white solid , the white solid was vacuum-dried at 45-50°C for 10-12 hours to obtain the crude product of fludarabine phosphate with a purity of 99.1% and a yield of 74.2%.
[0034] Add the fludarabine phosphate crude product obtained by the above pre...
Embodiment 2
[0036] Under stirring conditions, put fludarabine (10g), triethyl phosphate (90mL), and phosphorus oxychloride (6mL) in a low-temperature circulation pump at -5 to -10°C, while keeping the internal temperature at -5 to -1°C; Stir the reaction at -5~-1°C for 15 hours; when the amount of fludarabine is less than 2% in the HPLC detection area, the reaction is considered complete, and then pure water (50mL) is added under stirring The mixture was washed with dichloromethane, the obtained aqueous phase was adjusted to pH = 3 with 50% NaOH solution, concentrated under reduced pressure at 35-40°C, refrigerated for crystallization, filtered, and the filter cake was washed with a small amount of ethanol and water to obtain a white solid. The white solid was vacuum-dried at 45-50°C for 10-12 hours to obtain the crude product of fludarabine phosphate with a purity of 99.2% and a yield of 73.3%.
[0037] Add the fludarabine phosphate crude product obtained by the above preparation method ...
Embodiment 3
[0039] Under stirring conditions, put fludarabine (10g), triethyl phosphate (150mL), and phosphorus oxychloride (12mL) in a low-temperature circulation pump at -5 to -10°C, while maintaining an internal temperature of -5 to -1°C; Stir the reaction at -5~-1°C for 15 hours; when the amount of fludarabine is less than 2% in the HPLC detection area, the reaction is considered complete, and then pure water (200mL) is added under stirring The mixture was washed with dichloromethane, the obtained aqueous phase was adjusted to pH = 3 with 50% NaOH solution, concentrated under reduced pressure at 35-40°C, refrigerated for crystallization, filtered, and the filter cake was washed with a small amount of ethanol and water to obtain a white solid. The white solid was vacuum-dried at 45°C-50°C for 10-12 hours to obtain the crude product of fludarabine phosphate with a purity of 99.1% and a yield of 71%.
[0040]Add the fludarabine phosphate crude product obtained by the above preparation me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com