Method for preparing flame-retardant waterborne polyurethane containing phosphaphenanthrene and/or phenyl phosphate groups
A phenyl phosphate, water-based polyurethane technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. Base chain, unable to meet the requirements of bonding hardness, heat resistance and mechanical properties, etc., to achieve the effect of lasting flame retardant properties, uniform distribution, and difficult migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
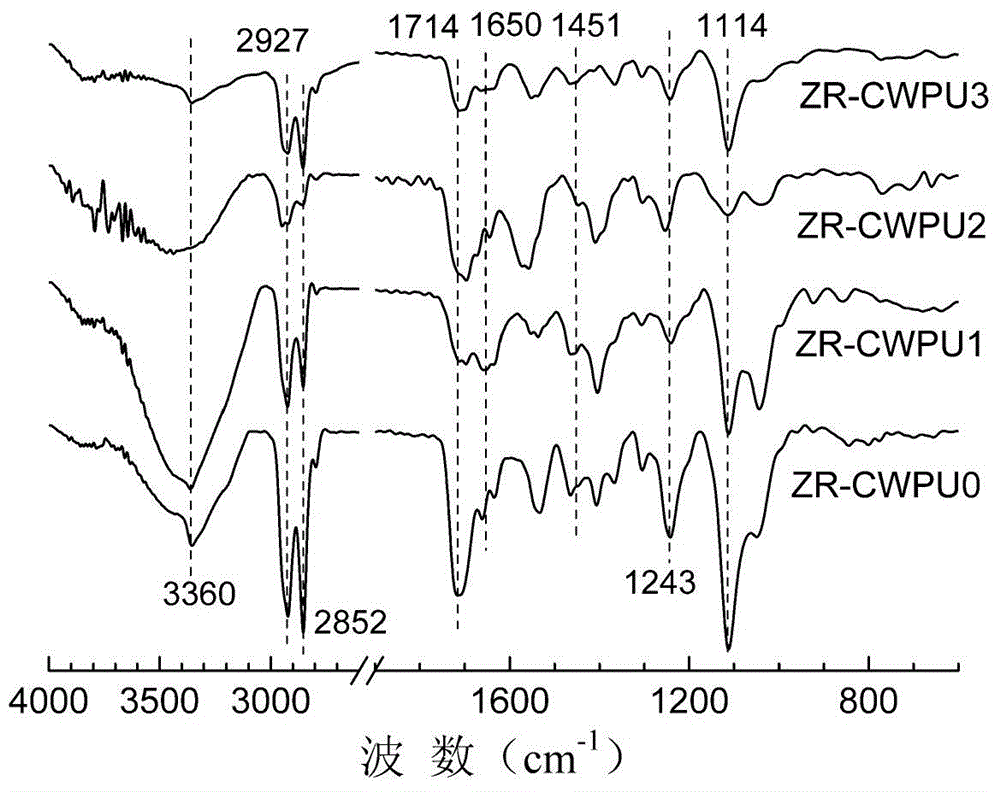
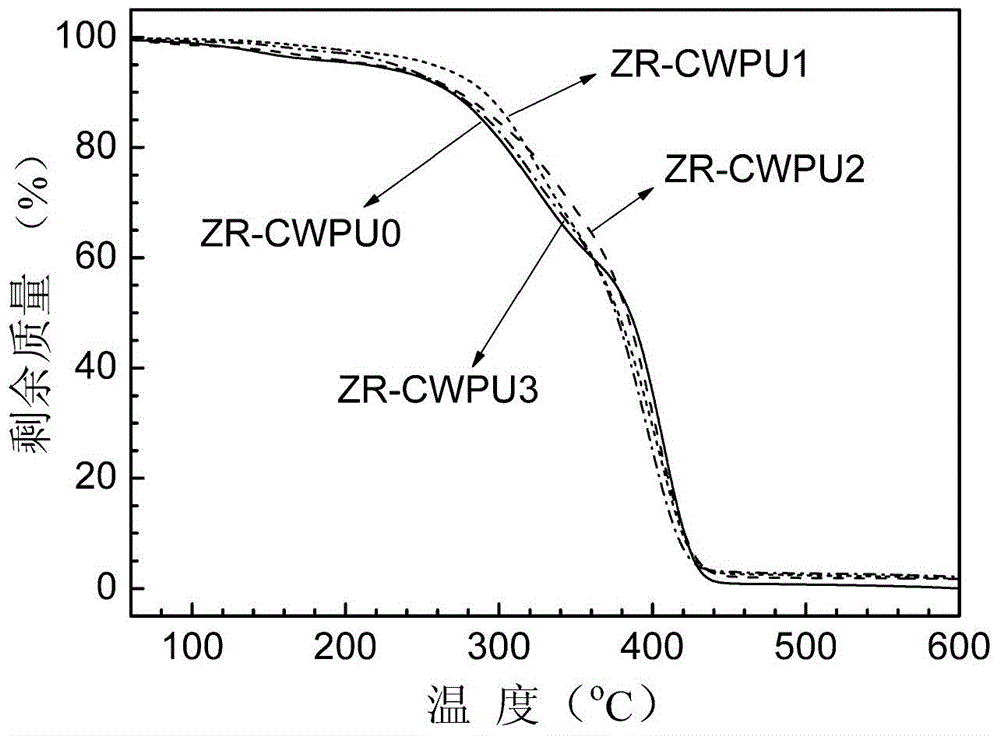
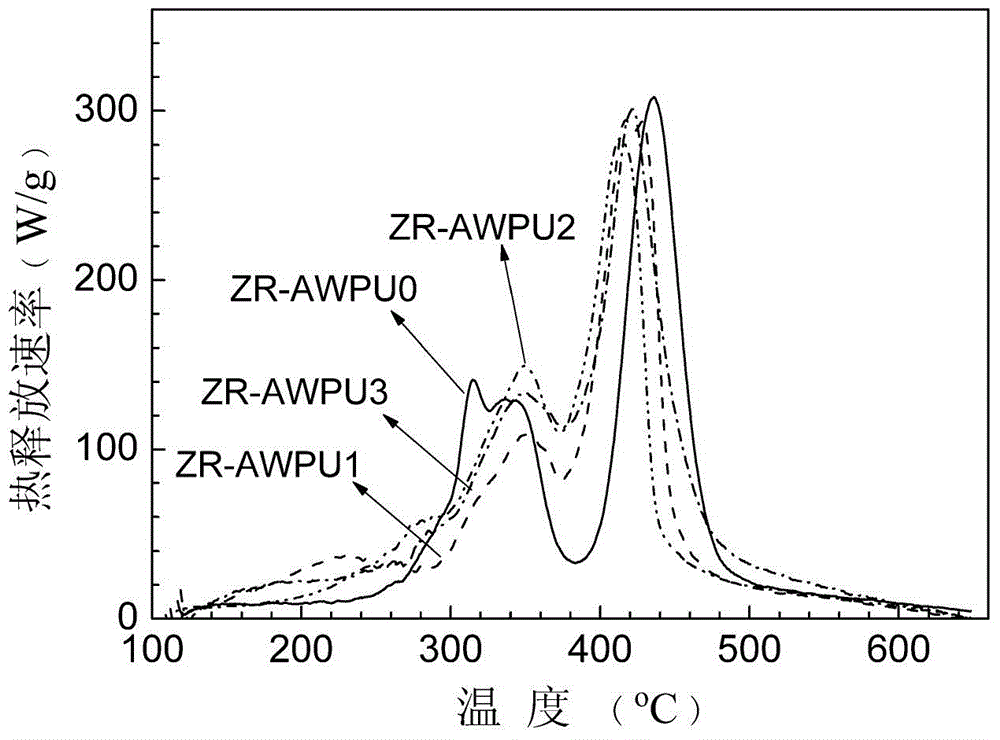
Image
Examples
Embodiment 1
[0041] Dissolve 15 grams of 1,6-hexanediol in 50 mL of ethyl acetate, add 12.8 grams of triethylamine into a 500 mL three-neck flask, and place the reaction system in an ice-water bath. Dissolve 13.4 grams of phenyl dichlorophosphate in 50 mL of ethyl acetate to form a solution, stir and drop the solution into the three-necked flask within 2 hours; the reaction system continues to react in an ice-water bath for 12 hours; The precipitate was filtered, and the filtrate was distilled under reduced pressure to remove the solvent to obtain a flame-retardant dihydric alcohol dihydroxyhexyl phenyl phosphate (ZR-Diol A) with a symmetric phenyl phosphate group structure, and the yield was 85%. The structural formula of ZR-Diol A is:
[0042]
[0043] 50 grams of PPG (M n =2000) was added to a 500mL three-necked flask, dehydrated at 110°C for 1 hour and then cooled to 50°C; 18.3 grams of TDI was added to the three-necked flask, stirred and reacted at 80°C for 2 hours, then added 5.0...
Embodiment 2
[0045] Add 10g of DOPO, 0.03g of triethylamine and 8.6g of MEDB into a 500mL three-necked flask, then add 100mL of toluene as a solvent, stir and heat up to 110°C for 24 hours; after the reaction system is cooled to room temperature, precipitate and filter , dried to obtain intermediate product A; product A was transferred to a three-necked flask, and a mixed solution of tetrahydrofuran and hydrochloric acid (volume ratio 1:1) with a concentration of 1M was added dropwise, wherein the ratio of the amount of DOPO to hydrochloric acid was 2.3:1 ; Reaction at room temperature for 1 hour, after vacuum distillation to remove tetrahydrofuran and water, flame retardant diol II containing phosphaphenanthrene group was obtained with a yield of 85%.
[0046] 80 grams of PCL (M n =1000) into a 500mL three-necked flask, dehydrated at 110°C for 1 hour and then cooled to 50°C; took 52.5 grams of IPDI and added it to the three-necked flask, stirred and reacted at 80°C for 2 hours, then added...
Embodiment 3
[0051] Add 100 grams of DOPO, 50 grams of diethanolamine and 400 mL of water into the reactor, and raise the temperature to 80°C under nitrogen protection and stirring. After the DOPO is dissolved, add 50 grams of formaldehyde with a concentration of 30-40% dropwise within half an hour. The temperature was raised to 120°C, and the reflux reaction was maintained for 3 hours; part of the water was extracted under 60°C and 0.01MPa vacuum conditions (the remaining volume of the reaction solution was controlled to be 100mL); the remaining liquid was recrystallized at 5°C for 96 hours to obtain white crystals; The white crystals were repeatedly rinsed with 100 mL of water and 100 mL of chloroform three times, then placed in a vacuum oven and dried to constant weight to obtain flame retardant diol I with a yield of 81%.
[0052] 50.00 grams of PTMG (M n =2000) into a 500mL three-necked flask, dehydrated at 115°C for 1 hour and then cooled to 50°C; took 22.00 g of IPDI and added it to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com