Method for synthesizing sucrose fatty acid ester
A technology for the synthesis of fatty acid sugar esters, which is applied in the field of enzymatic synthesis, can solve the problems of insoluble polar opposite substrate environment, polluting enzyme activity, etc., and achieve the goal of simplifying the purification process of sugar esters, improving efficiency, and increasing space-time yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
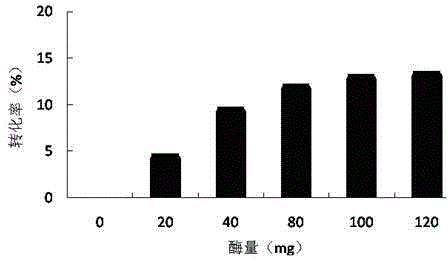
[0046] Embodiment 1, optimize enzyme amount
[0047] Substrate molar ratio (VL / Glc, Glc: 0.3M) 1:1, water content 0.08% (concentrated for 24h), solvent volume 1mL, molecular sieve 100mg, reaction temperature 40°C, enzyme volume 20mg, 40mg, 60mg, 80mg , 100mg and 120mg, under 300rpm shaker, react for 24h.
[0048] The ionic liquid is pre-concentrated in a vacuum centrifugal dryer at 60°C for 24 hours, and the vinyl laurate is dried for more than 2 weeks through 4 A molecular sieves (the same below).
[0049] From figure 1 It can be seen that the amount of enzyme does not increase significantly after 100 mg. Considering the factors of conversion rate and cost, the amount of enzyme is selected to be 100 mg.
Embodiment 2
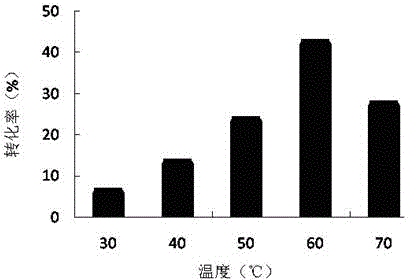
[0050] Embodiment 2, optimize reaction temperature
[0051] Substrate molar ratio (VL / Glc, where Glc is 0.3M) 1:1, solvent volume 1mL, water content 0.08% (concentrated for 24h), enzyme volume 100mg, reaction temperatures were 30°C, 40°C, 50°C, 60°C ℃ and 70 ℃, under the shaker at 300rpm, react for 24h.
[0052] From figure 2 It can be seen from the figure that the conversion rate increases gradually before the reaction temperature is 60°C, but decreases after 60°C, so the optimal reaction temperature is 60°C.
Embodiment 3
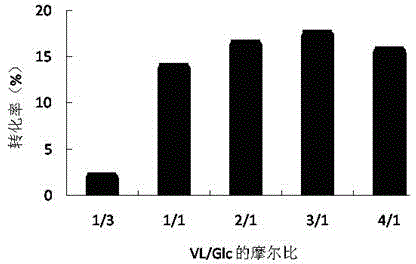
[0053] Embodiment 3, optimize mol ratio
[0054] The amount of anhydrous glucose is 0.054g (0.3M), the amount of solvent is 1mL, the water content is 0.08% (concentrated for 24h), the molecular sieve is 100mg, the enzyme amount is 100mg, and the concentrations of vinyl laurate are: 0.1, 0.3, 0.6, 0.9 and 1.2M (that is, the VL / Glc molar ratios are: 1:3, 1:1, 2:1, 3:1, and 4:1), the reaction temperature is 40°C, and the reaction is 24h under the shaker at 300rpm.
[0055] From image 3 It can be seen that when the molar ratio is 3:1, the conversion rate of glycolipid is the highest.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com