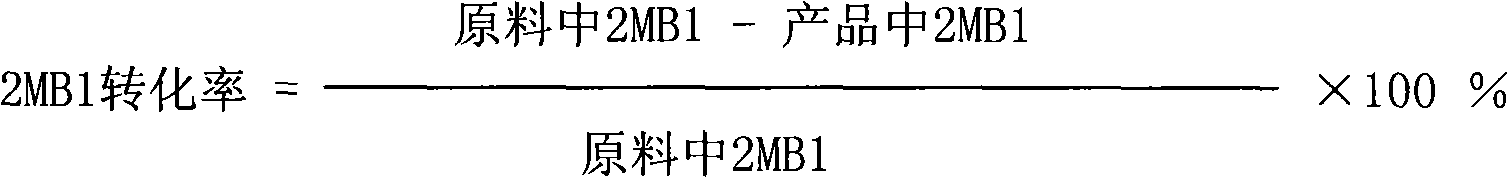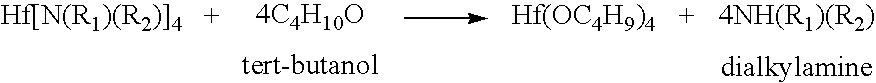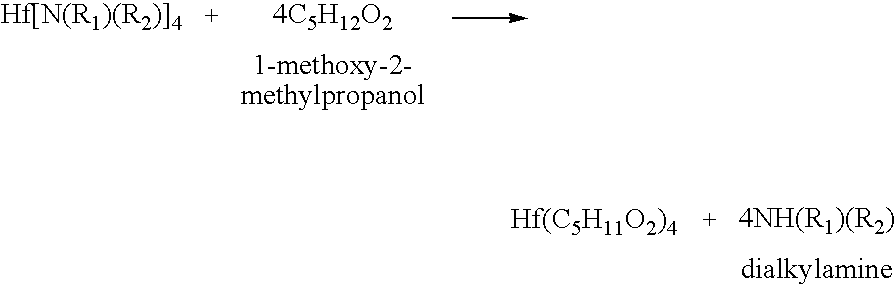Patents
Literature
54 results about "Tertiary amyl alcohol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Manufacturing method of methylamine lead iodine perovskite solar cell
ActiveCN107359246AImprove securityWon't breakSolid-state devicesSemiconductor/solid-state device manufacturingPerovskite solar cellAnti solvent
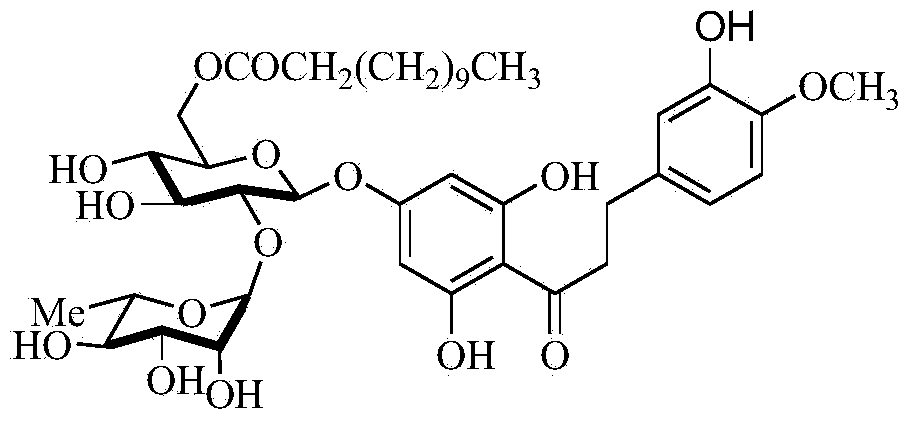
The invention relates to the field of solar cells, and provides a manufacturing method of a methylamine lead iodine perovskite solar cell. A photosensitive layer is a methylamine lead iodine perovskite film. The methylamine lead iodine perovskite film is manufactured by the spin coating technology and the anti-solvent dropping and cleaning technology with combination of the solvent annealing technology. The spin coating technology refers to the process that a methylamine lead iodine perovskite CH3NH3PbI3 precursor solution is spin-coated on a PEDOT:PSS film. The anti-solvent cleaning technology refers to the process that the anti-solvent amyl alcohol is dropped in the process of spin-coating the CH3NH3PbI3 methylamine lead iodine perovskite precursor solution so that the perovskite is enabled to be quickly crystalized and precipitated, wherein the anti-solvent amyl alcohol is sec-amyl alcohol or tertiary amyl alcohol. The adopted preparation technology is simple so that the methylamine lead iodine perovskite solar cell of high efficiency and great repeatability without the hysteresis phenomenon can be prepared.
Owner:TAIYUAN UNIV OF TECH
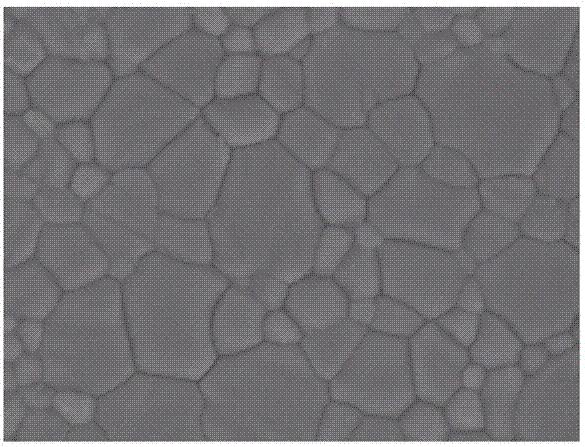
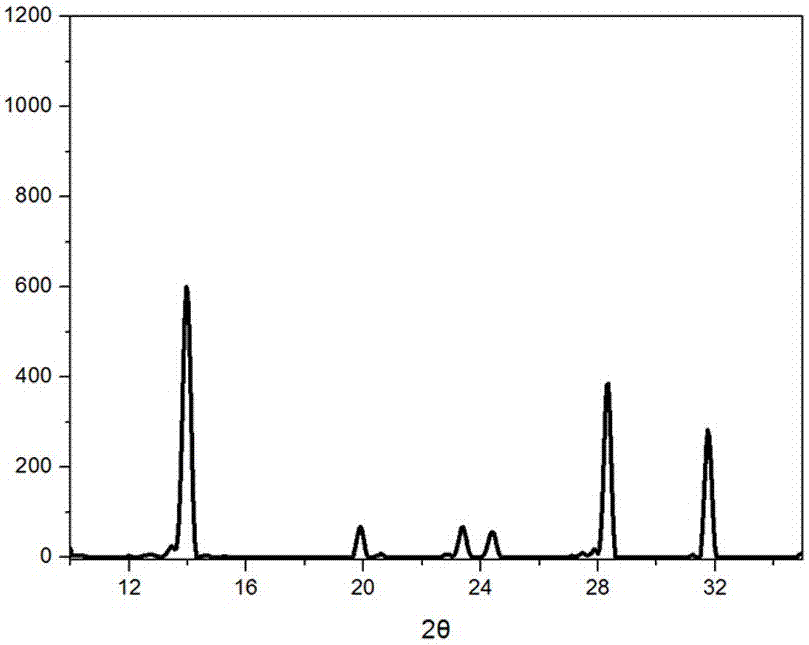
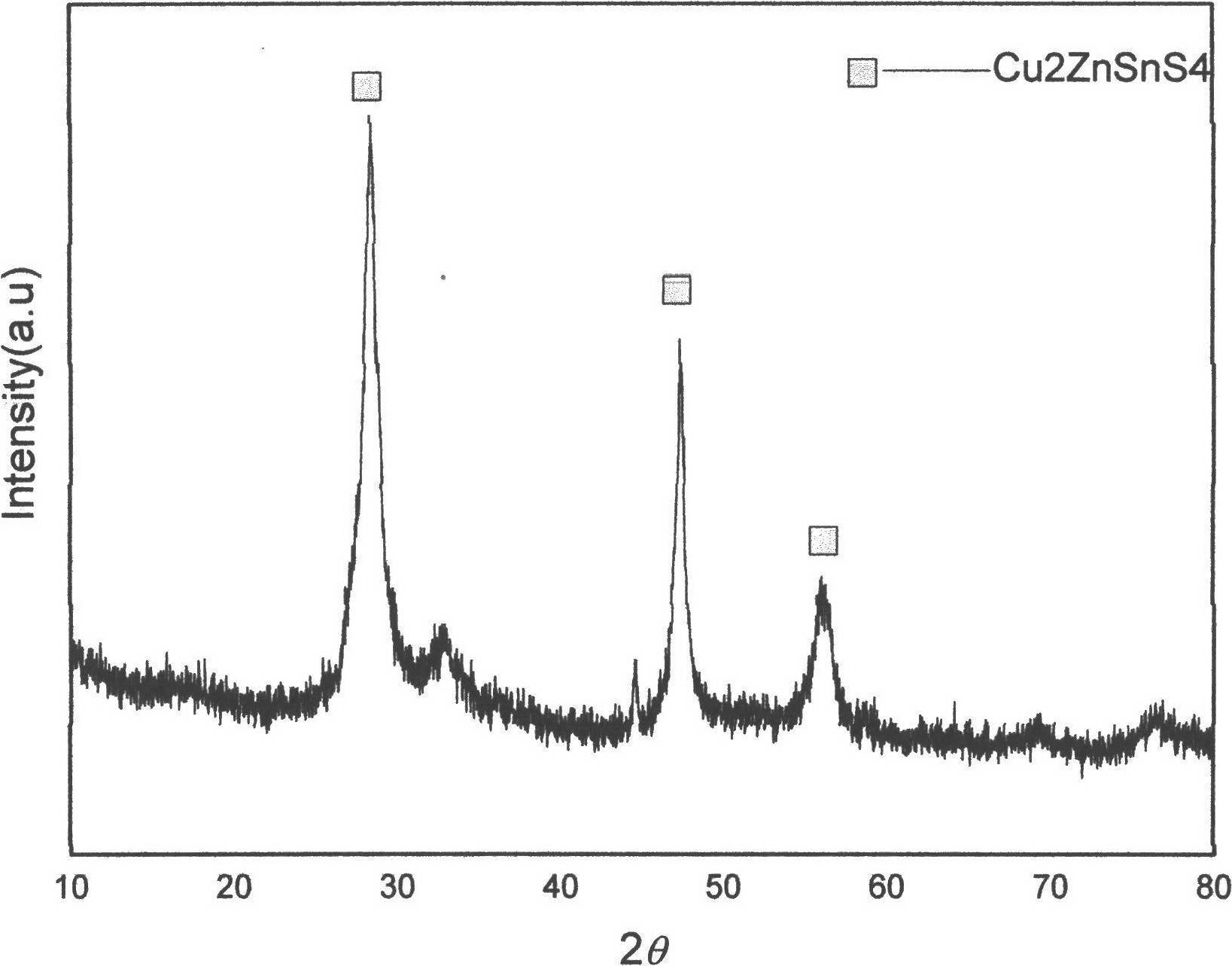
Method for preparing CZTS (Copper Zinc Tin Sulfide) (Se) series nanometer powder by low-temperature mechanical alloying
InactiveCN102642818AAvoid introducingReaction raw materials are readily availableTin compoundsSelenium/tellurium compundsIsobutanolHexamethylenediamine
The invention discloses a method for preparing CZTS (Copper Zinc Tin Sulfide) (Se) series nanometer powder by low-temperature mechanical alloying. Elementary substances Cu powder, Zn powder, Sn powder and S (Se) powder are added into a ball-milling tank according to a certain mole ratio, an alcohol and amine mixed liquor is used as a process control agent, ball milling is carried out according to a rated ratio of grinding media to material, a set rotational speed and ball milling time, and a ball-milled product is centrifugally washed and dried to obtain a target product. In the raw materials, elementary substances sulfur powder and selenium powder can be exchanged in any mole ratio; and the process control agent is the mixed liquor of alcohol and amine with the volume ratio of 1-20:1, the alcohol is one of ethanol, ethylene glycol, normal butanol, isobutanol, isoamylol, tertiary amyl alcohol and glycerol, and the amine is one of ethanediamine, iso-butylamine, diisopropylamine, hexamethylenediamine and triethylamine. The method disclosed by the invention has the advantages of easy obtainment of raw materials, pure products, low energy consumption, easy control in product shape and appearance, simple process and the like, and is suitable for industrial production.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
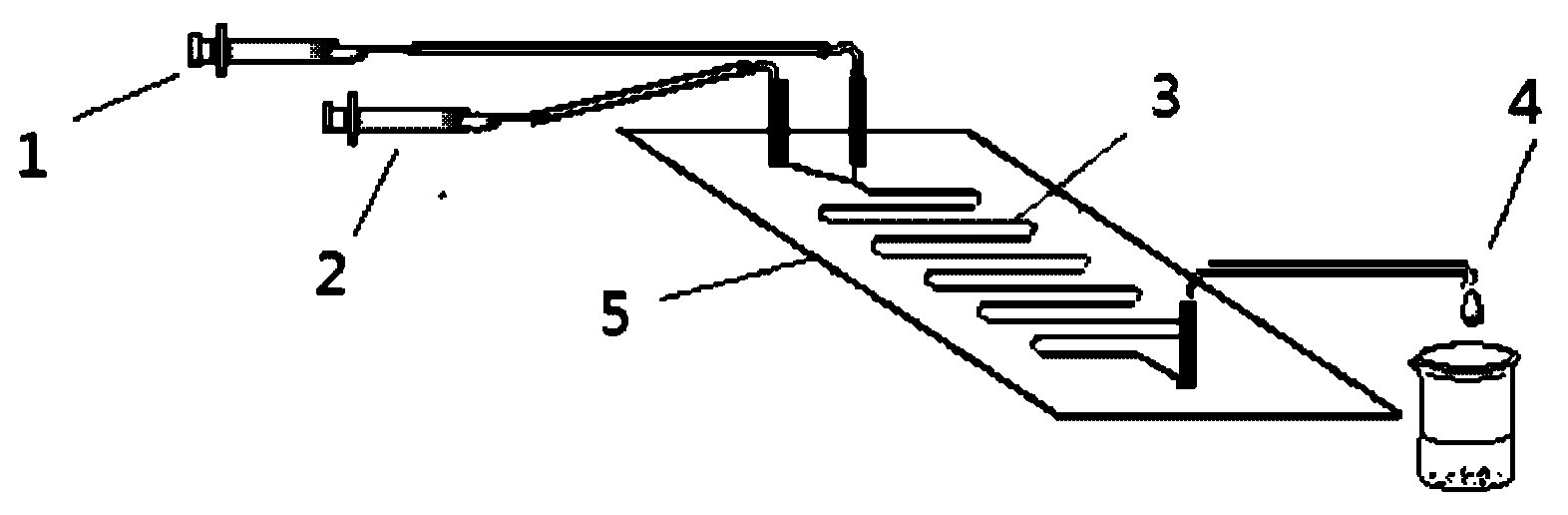
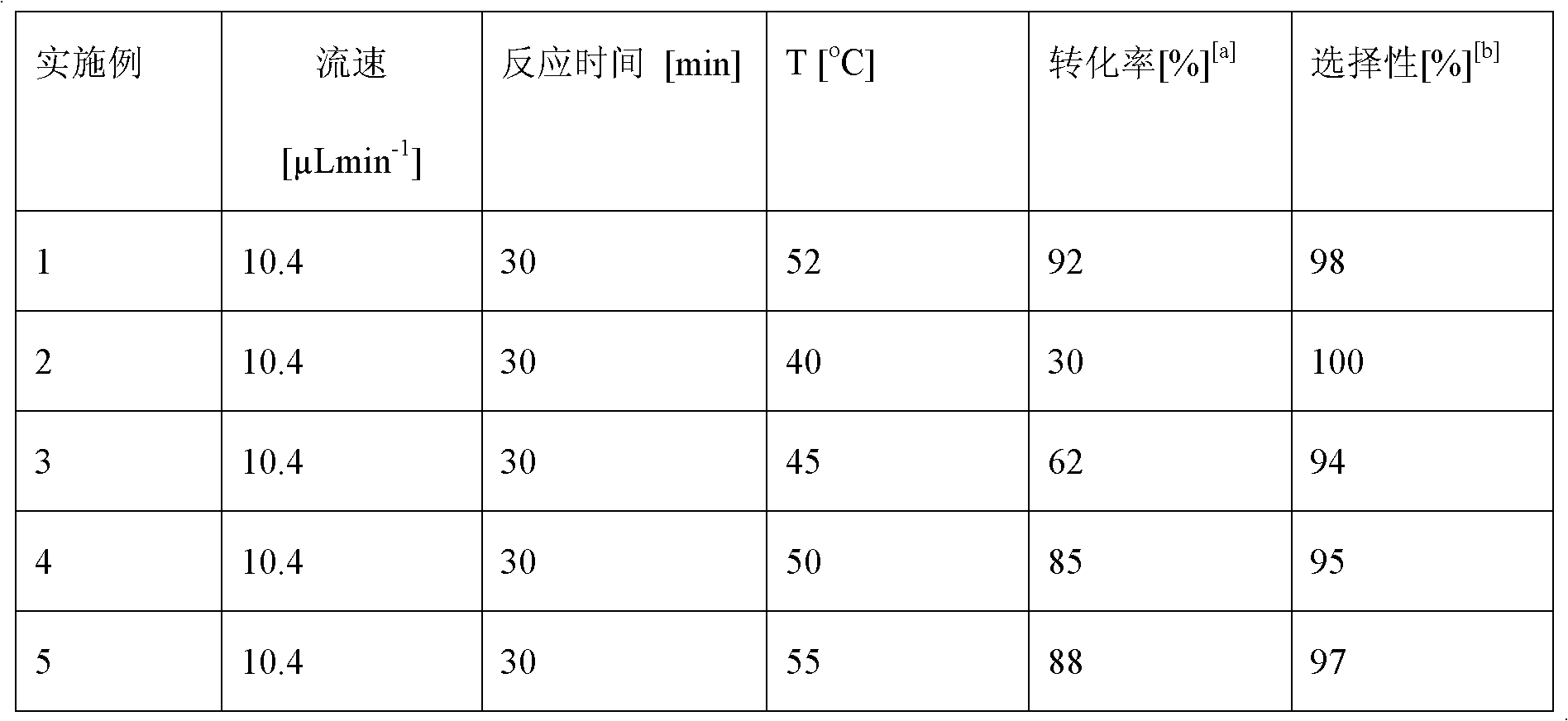
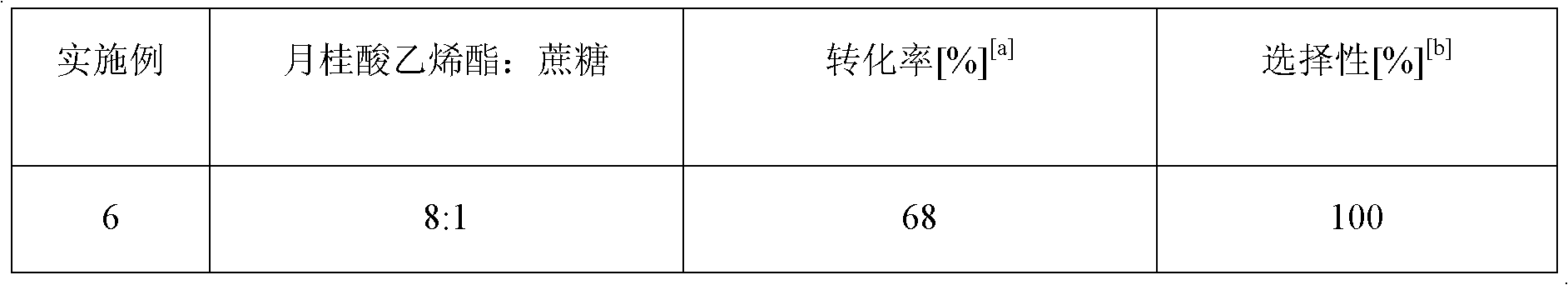
Method for on-line synthesizing saccharose-6-laurate by lipase catalysis
ActiveCN103184256AReduce usageShort reaction timeBioreactor/fermenter combinationsBiological substance pretreatmentsMicrofluidic channelSucrase
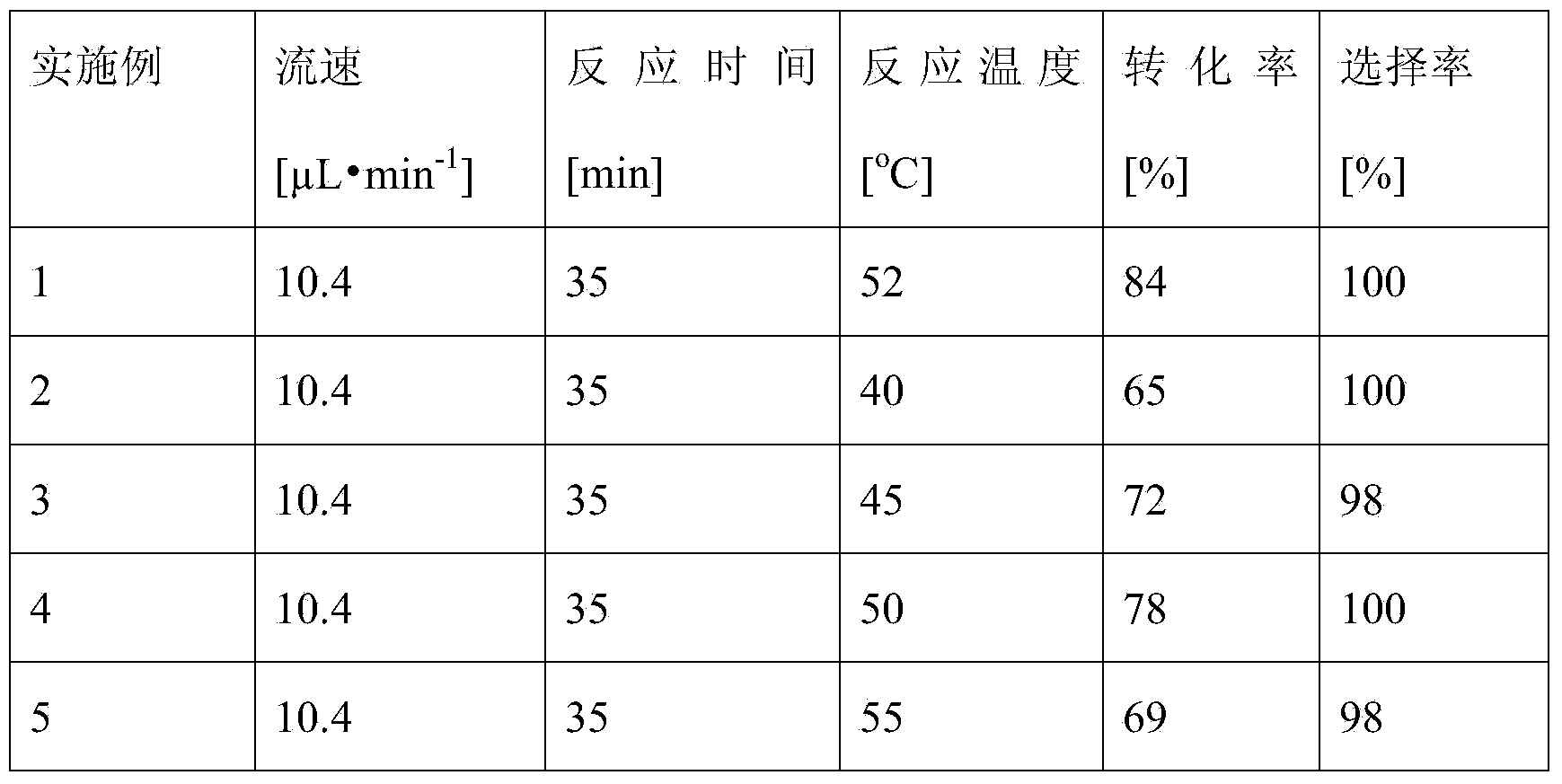
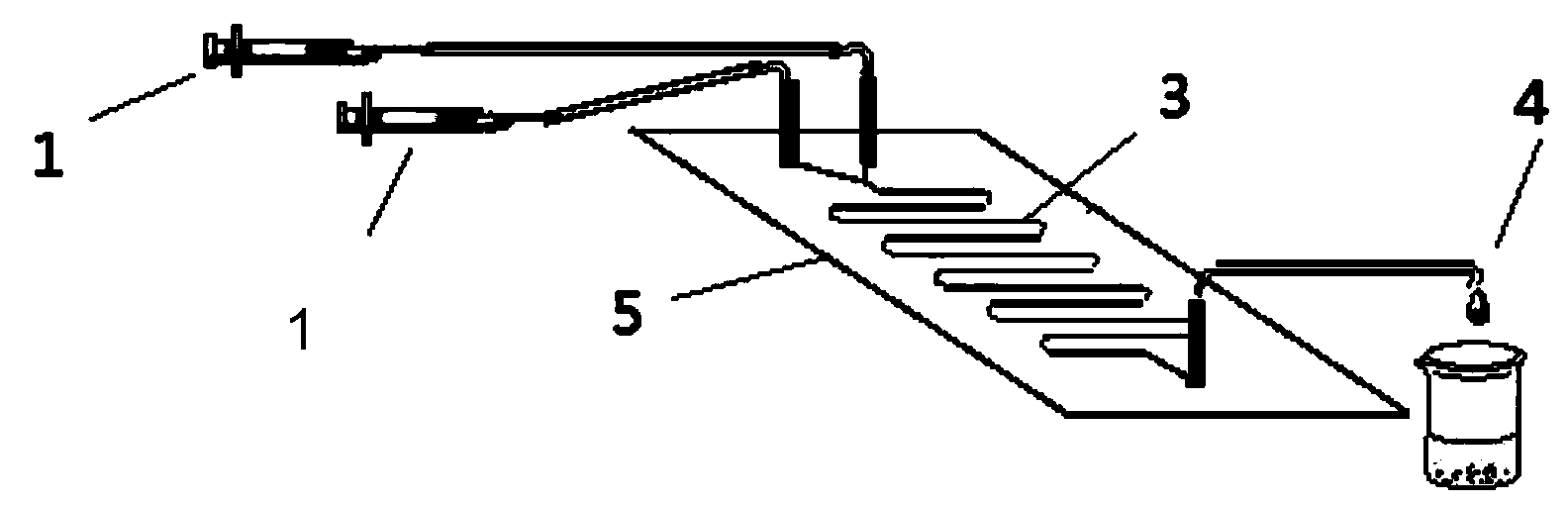
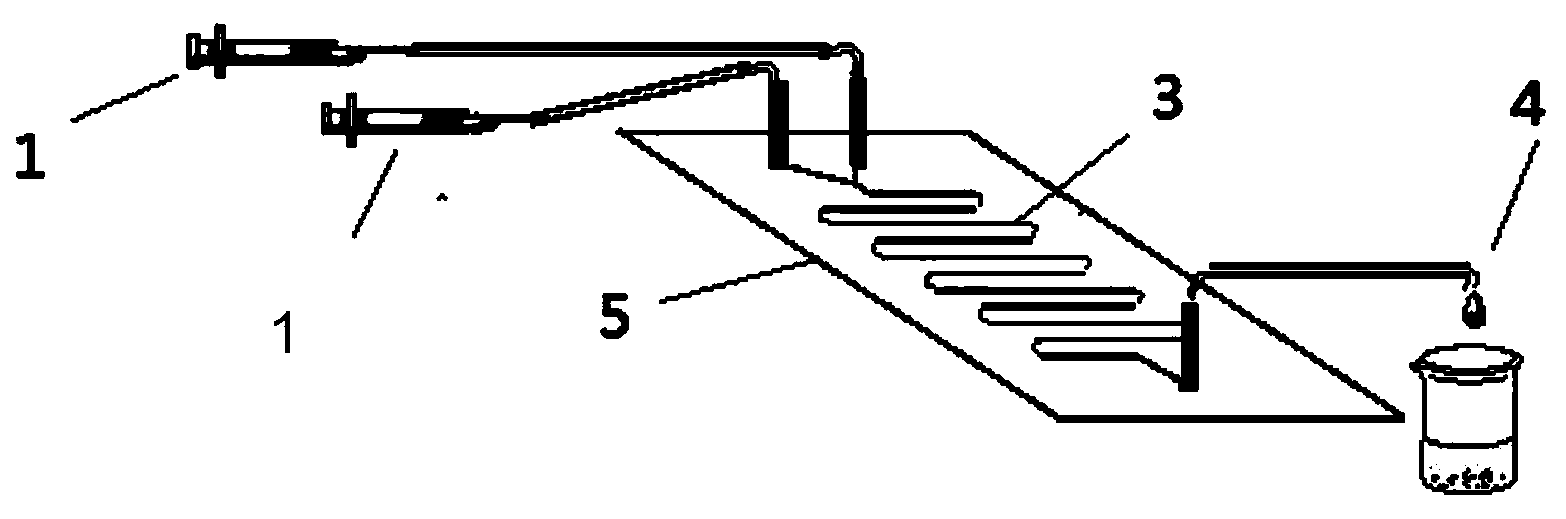
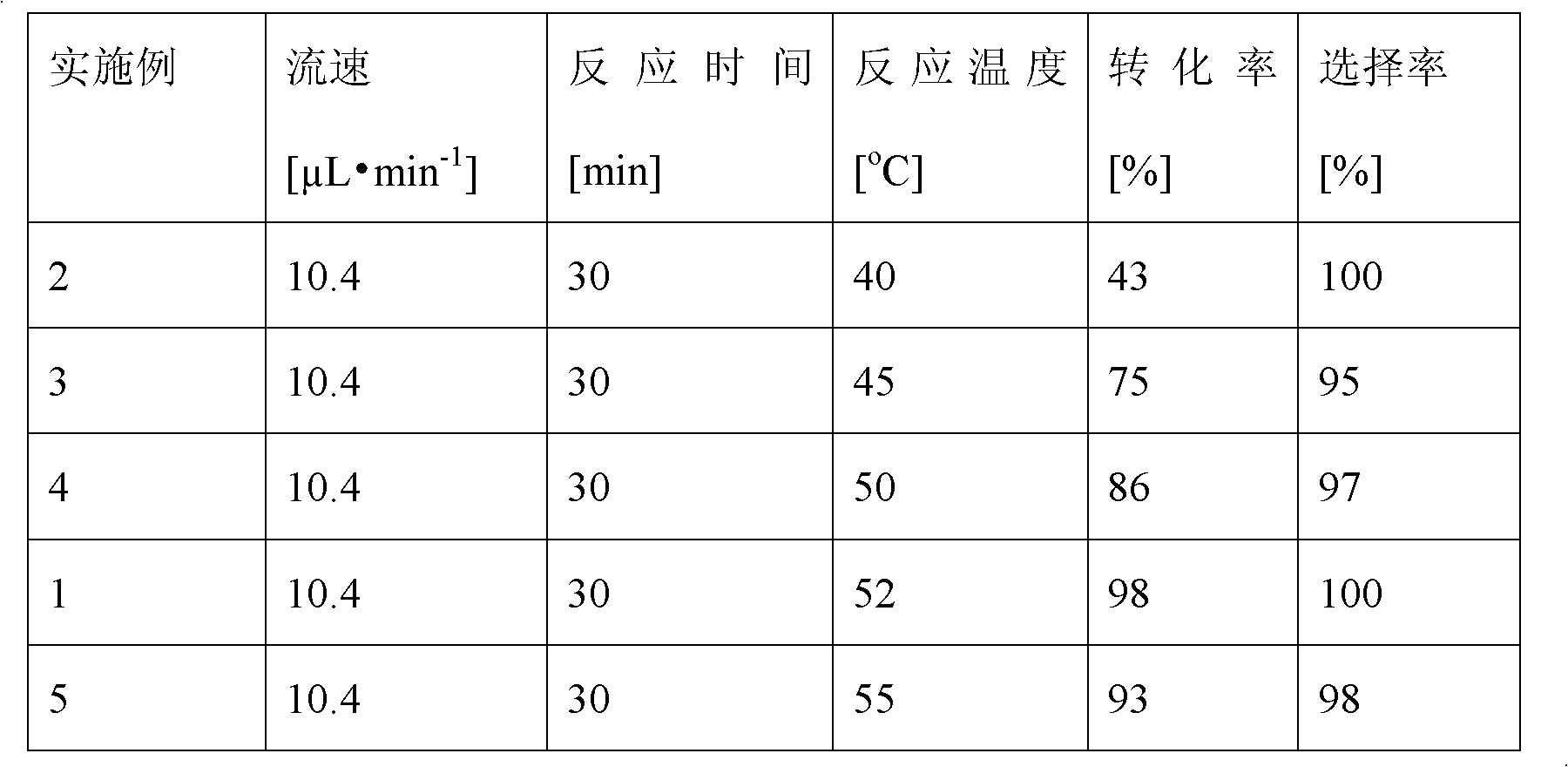
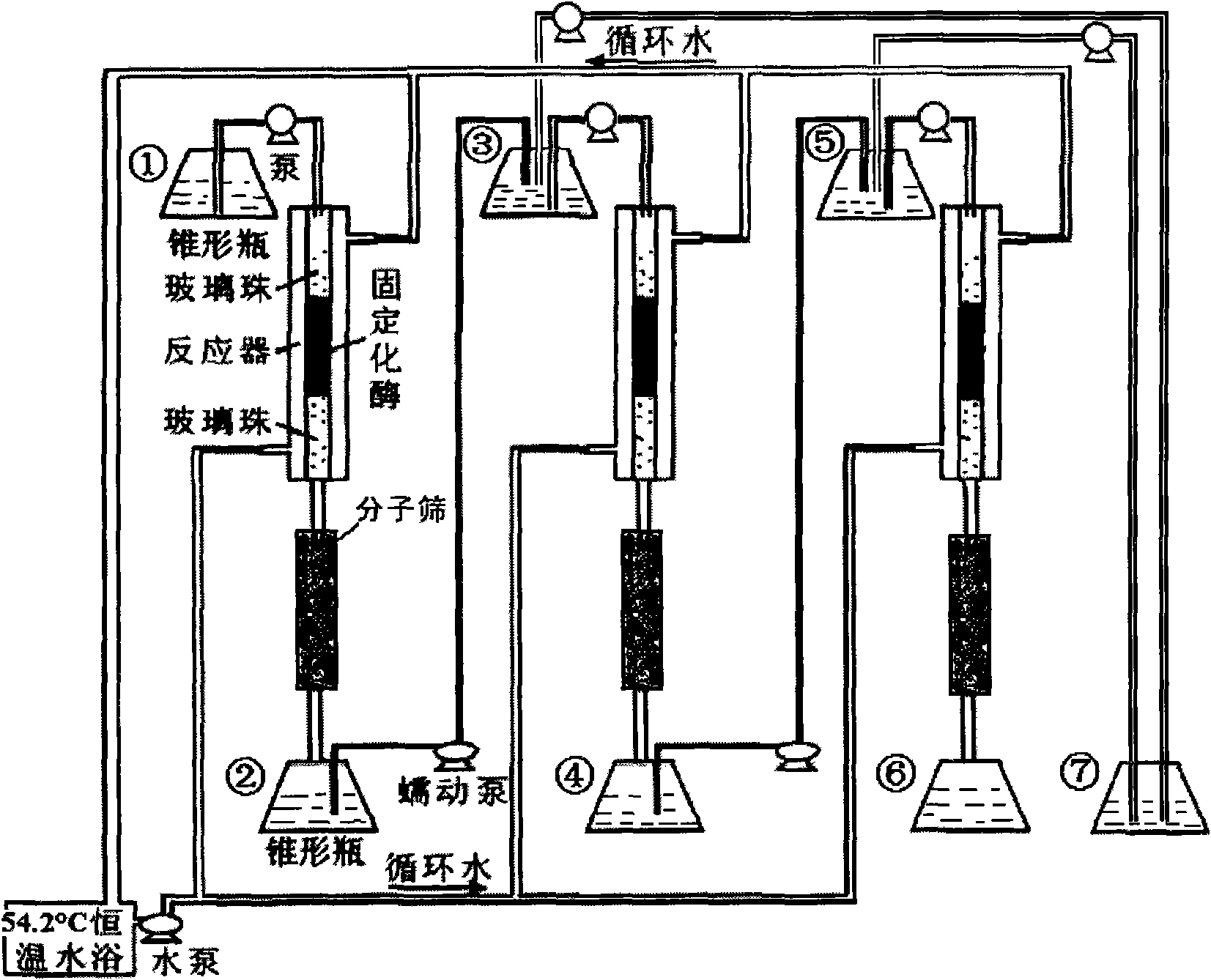
The invention provides a method for on-line synthesizing saccharose-6-laurate by lipase catalysis, comprising: using saccharose and vinyl laurate, with a mol ratio of 1:8-12, as raw materials, using 0.5-10g of Lipozyme TLIM as a catalyst, and using a mixed solvent of tertiary amyl alcohol and DMSO as a reaction solvent, uniformly filling Lipozyme TLIM in a reaction channel of a microfluidic channel reactor, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the length of the reaction channel is 0.5-1.0m; continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction under 40-55 DEG C for 20-35min, on-line collecting the reaction solution, and then obtaining the saccharose-6-laurate after conventional post-treatment on the reaction solution. The method of the invention has advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
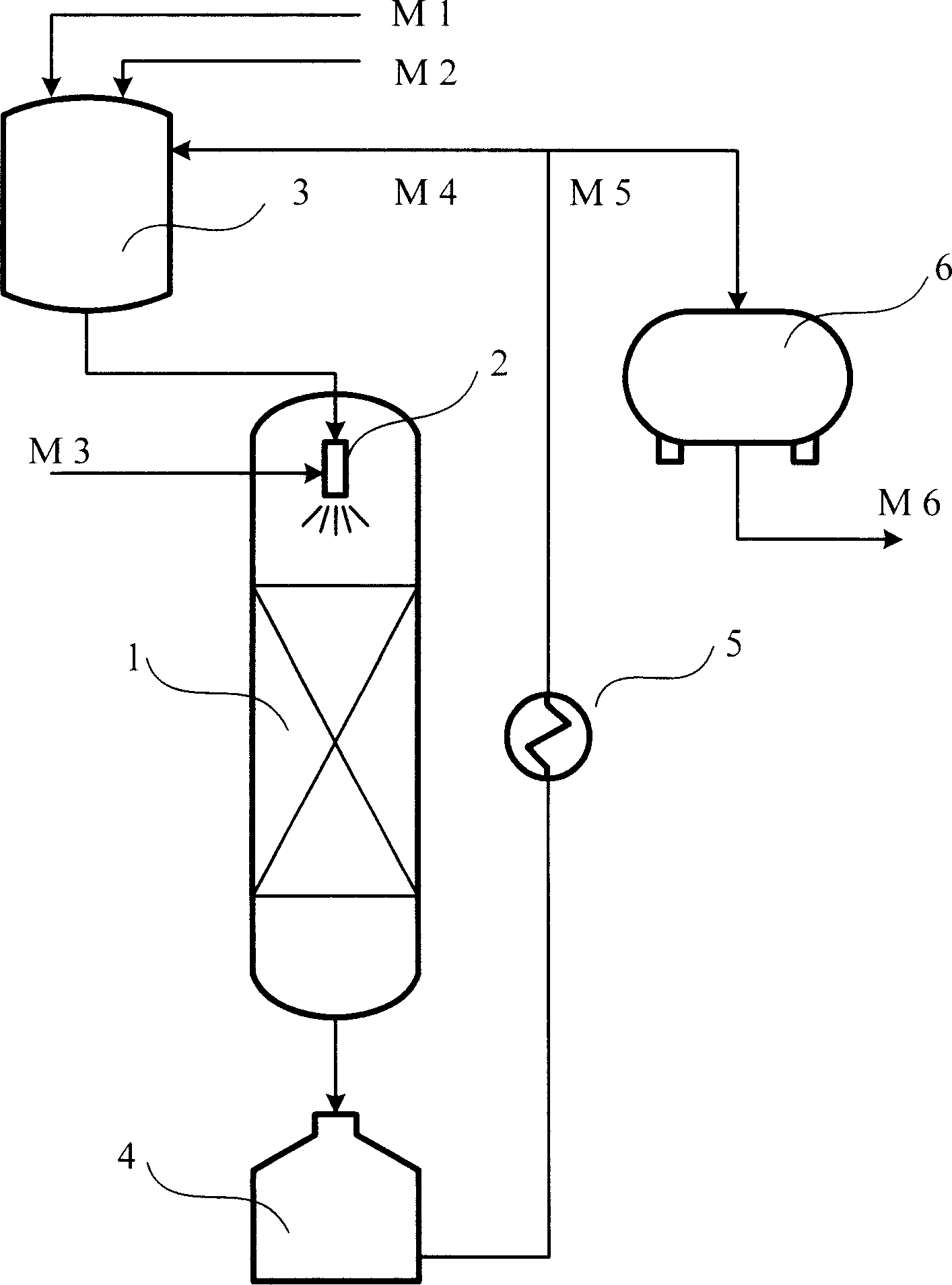
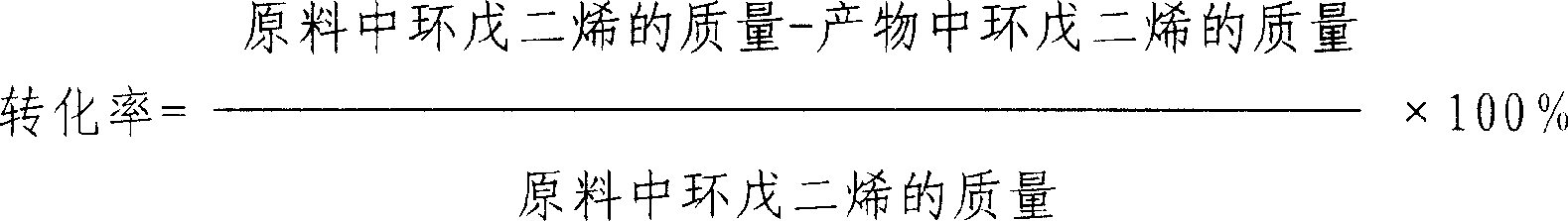
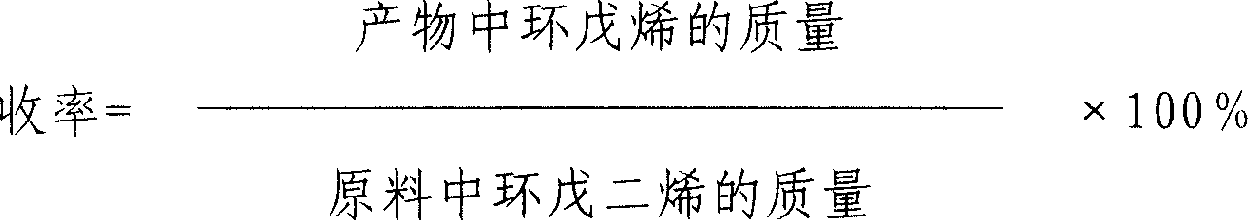
Method of preparing cyclopentene by continuous hydrogenation of cyclopentadiene
InactiveCN1911877AWell mixedImprove mass transfer efficiencyHydrocarbon by hydrogenationCyclopenteneFixed bed
The continuous cyclopentadiene hydrogenating process to prepare cyclopentene is one continuous catalytic hydrogenation reaction of the mixture of cyclopentadiene, solvent and hydrogen in a fixed bed catalyst bed. The catalyst includes gamma-Al2O3 as carrier and Pa as active component. The solvent is any one of benzene, toluene, cyclohexane, ethanol, methanol, tertiary amyl alcohol and tertiary butyl alcohol. The molar ratio between cyclopentadiene and hydrogen is 1 to 1.5-3.5, and the reaction system has pressure of 0.9-1.5 MPa and temperature of 30-55 deg.c. The reacted liquid is discharged partially and externally circulated partially with the weight ratio being 1 to 6-12, and the externally circulated liquid after being cooled is mixed with cyclopentadiene material and solvent before being mixed with hydrogen in venturi ejector, atomized into gas-liquid mixture and reacted in the catalyst bed. The catalyst load is 4-7 / hr accounted in liquid phase material, and the reacted heat is transferred via external circulation.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD +1
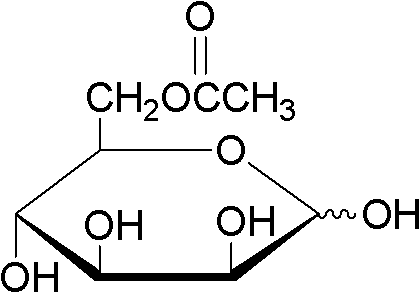
Method for synthesizing cane sugar-6-acetic ester by using lipase for catalyzing
The invention discloses a method for synthesizing a cane sugar-6-acetic ester by using lipase for catalyzing. The method comprises the following steps of: based on 72,000 to 150,000 enzyme activity unit enzyme in 100 ml buffer solution, adding the lipase into buffer solution with a pH value of between 8.1 and 8.7 to obtain enzyme solution; adding an organic solvent into the enzyme solution; adding cane sugar until the cane sugar in reaction solution is saturated; stirring until the cane sugar is solved and adding an acetic ester compound; reacting at the temperature of between 40 and 65 DEG C with stirring; adding the acetic ester compound after reacting for 11 to 13 hours; ending an reaction after reacting totally for 23 to 25 hours; and washing the reaction product with distilled water, decompressing and distilling to obtain cane sugar-6-acetic ester, wherein the acetic ester compound is vinyl acetate or isopropenyl acetate; the volume ratio of the enzyme solution to the organic solvent is 0.1-1:100; and the organic solvent is tertiary amyl alcohol, sec-butyl alcohol or tetrahydrofuran. The organic solvent is selected and the water content of a reaction system is less than 1.0 percent, so that the purity of the obtained high-purity cane sugar-6-acetic ester is over 75 percent, and 99 percent in maximum.
Owner:ZHEJIANG UNIV OF TECH
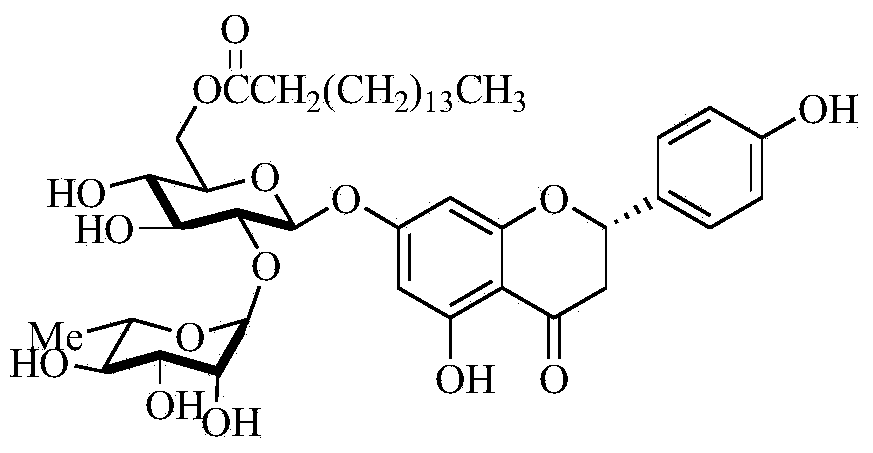
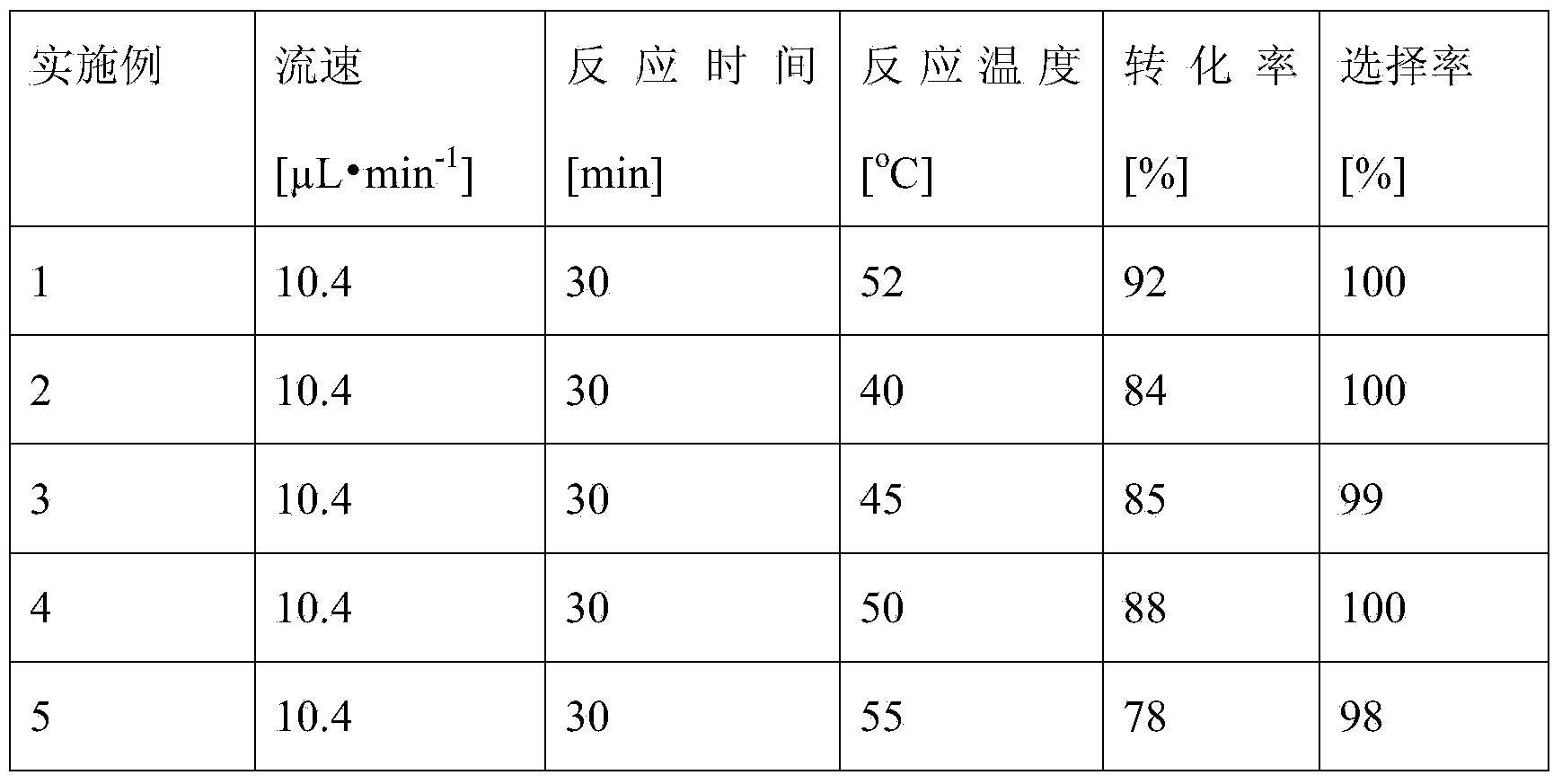
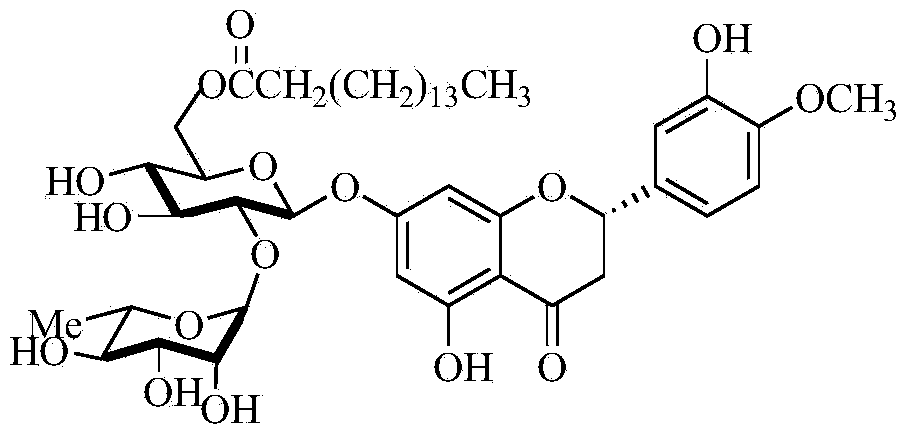
Method for synthesizing 6''-O-lauroyl-naringin ester on line by using lipase as catalyst
The invention discloses a method for synthesizing a 6''-O-lauroyl-naringin ester on line by using lipase as a catalyst, which comprises the following steps: by using naringin and vinyl laurate in a mole ratio of 1:(1-9) as raw materials, 0.5-1.0g of lipase Lipozyme RMIM as a catalyst and a tertiary amyl alcohol-DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme RMIM into a reaction channel of a microfluidic channel reactor, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, wherein the acylation reaction temperature is controlled at 40-55 DEG C, and the acylation reaction time is 15-35 minutes; and collecting the reaction solution on line, and carrying out conventional after-treatment on the reaction solution to obtain the 6''-O-lauroyl-naringin ester. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Synthesis of sucrose-6-fatty acid ester through selective catalysis of immobilized aspergillus oryzae lipase
InactiveCN102181494AHigh puritySolve many problems with harsh reaction conditionsFermentationChromatographic separationSucrose
The invention discloses synthesis of sucrose-6-fatty acid ester through selective catalysis of immobilized aspergillus oryzae lipase. A synthesis method comprises the following steps of: dissolving sucrose into a mixed solvent of dimethyl sulfoxide and tertiary amyl alcohol at constant temperature of 20-60 DEG C until the sucrose is completely dissolved; adding the immobilized aspergillus oryzae lipase and oscillating at the constant temperature; adding fatty acid vinyl ester for reaction; recording a conversion rate; filtering and separating the lipase serving as a catalyst and molecular sieve from a mixture after reaction is finished; cleaning and then drying under vacuum for future use; cooling a reaction product to room temperature; mixing a remaining part with water after reduced pressure distillation and organic phase extraction of residual reactants; extracting sucrose ester by using an organic solution; performing reduced pressure distillation, concentration and chromatographic separation on an organic phase; monitoring a combined product part through TLC (Thin Layer Chromatography); and performing recrystallization to obtain the sucrose-6-fatty acid ester. The method for preparing the sucrose-6-fatty acid ester through catalysis of the immobilized aspergillus oryzae lipase is environmentally-friendly and pollution-free, has high specificity, high yield and mild reaction conditions and is easy for separation and purification.
Owner:JK SUCRALOSE
Method for synthesizing sucrose fatty acid ester
InactiveCN104593445AHigh space-time yieldSimple purification processFermentationBulk chemical productionSolubilitySucrose
The invention discloses a method for synthesizing sucrose fatty acid ester. The method comprises the following steps: by taking an ionic liquid or a double solvent of the ionic liquid and an organic solvent as a reaction medium, adding fatty acid or fatty acid ester and saccharides with hydroxyl to the reaction medium at a mole ratio of (1:3)-(4:1), and mixing for 3-7 minutes; and then adding lipase, and reacting for 4-48 hours to prepare the sucrose fatty acid ester. According to the method, an environment-friendly high-efficiency reaction system for synthesizing sugar ester through an enzymic method is established by designing and optimizing a reaction condition in a pure ionic liquid system and a double-solvent system; especially, the solubility of a matrix and a product is enhanced through the ionic liquid and tertiary amyl alcohol in the double-solvent system, so that the space time yield of the sugar ester is enhanced, a purifying process of the sugar ester is simplified, and the efficiency is improved.
Owner:SHENZHEN UNIV
Method for synthesizing 6''-O-lauroyl-naringin dihydrochalcone ester on line by using lipase as catalyst
ActiveCN103667396AReduce usageShort reaction timeFermentationReaction temperatureMicrofluidic channel
The invention discloses a method for synthesizing a 6''-O-lauroyl-aringin dihydrochalcone ester on line by using lipase as a catalyst, which comprises the following steps: by using naringin dihydrochalcone and vinyl laurate in a mole ratio of 1:(1-9) as raw materials, 0.5-1.0g of lipase Lipozyme RMIM as a catalyst and a tertiary amyl alcohol-DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme RMIM into a reaction channel of a microfluidic channel reactor, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, wherein the acylation reaction temperature is controlled at 40-55 DEG C, and the acylation reaction time is 15-35 minutes; and collecting the reaction solution on line, and carrying out conventional after-treatment on the reaction solution to obtain the 6''-O-lauroyl-aringin dihydrochalcone ester. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
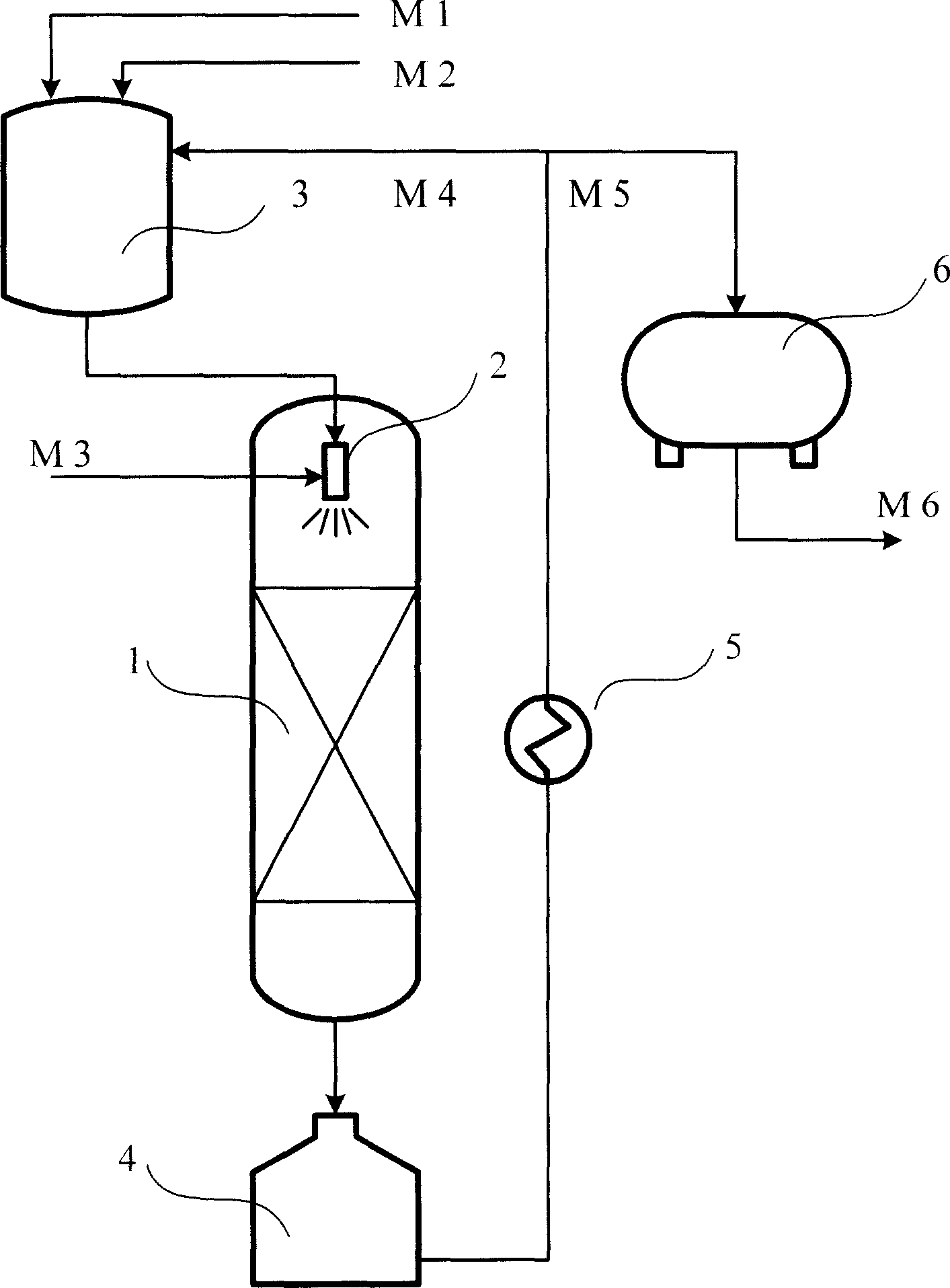
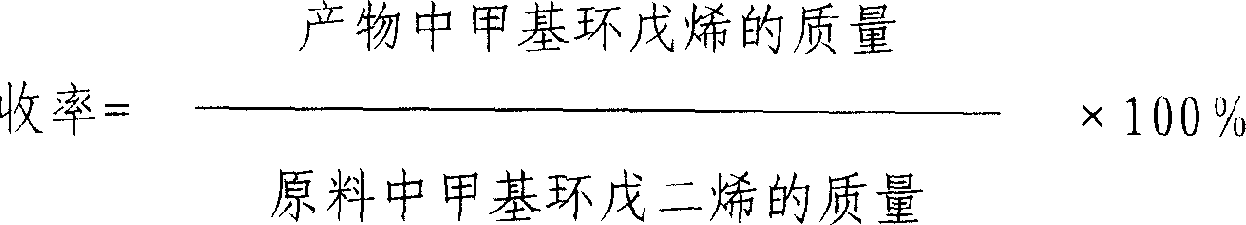
Method of preparing methyl cyclo penlene by continuous hydrogenation of methyl cyclo pentadiene
The continuous methyl cyclopentadiene hydrogenating process to prepare methyl cyclopentene is one continuous catalytic hydrogenation reaction of the mixture of methyl cyclopentadiene, solvent and hydrogen in a fixed bed catalyst bed. The catalyst includes gamma-Al2O3 as carrier and Pa as active component. The solvent is any one of benzene, toluene, cyclohexane, ethanol, methanol, tertiary amyl alcohol and tertiary butyl alcohol. The reaction system has pressure of 0.9-1.5 MPa and temperature of 60-100 deg.c. The reacted liquid is discharged partially and externally circulated partially with the weight ratio being 1 to 6-12, and the externally circulated liquid after being cooled is mixed with methyl cyclopentadiene material and solvent before being mixed with hydrogen in venturi ejector, atomized into gas-liquid mixture and reacted in the catalyst bed. The catalyst load is 3-6.0 / hr accounted in liquid phase material, and the reacted heat is transferred via external circulation. The present invention has high yield.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for synthesizing 6''-O-palmitoyl-neohesperidin dihydrochalcone ester on line by using lipase as catalyst
The invention discloses a method for synthesizing a 6''-O-palmitoyl-neohesperidin dihydrochalcone ester on line by using lipase as a catalyst, which comprises the following steps: by using neohesperidin dihydrochalcone and vinyl palmitate in a mole ratio of 1:(1-12) as raw materials, 0.5-1.0g of lipase Lipozyme RMIM as a catalyst and a tertiary amyl alcohol-DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme RMIM into a reaction channel of a microfluidic channel reactor, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, wherein the acylation reaction temperature is controlled at 40-55 DEG C, and the acylation reaction time is 20-40 minutes; and collecting the reaction solution on line, and carrying out conventional after-treatment on the reaction solution to obtain the 6''-O-palmitoyl-neohesperidin dihydrochalcone ester. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Method for synthesizing 6''-O-palmitoyl-naringin ester on line by using lipase as catalyst
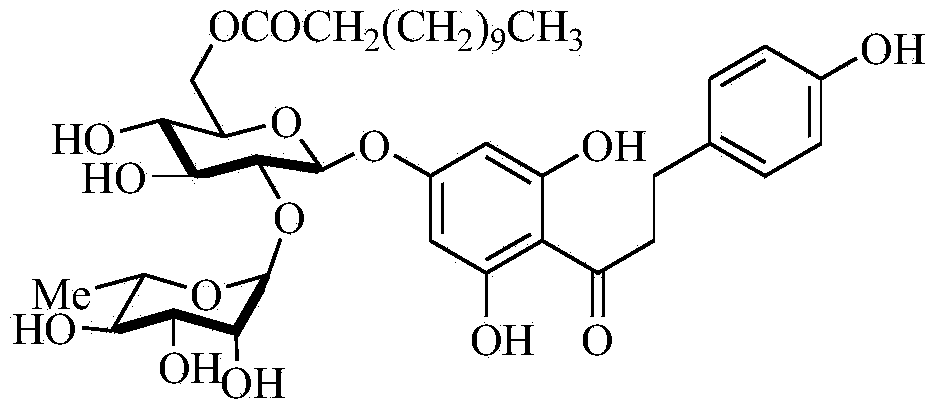
The invention discloses a method for synthesizing a 6''-O-palmitoyl-naringin ester on line by using lipase as a catalyst, which comprises the following steps: by using naringin and vinyl palmitate in a mole ratio of 1:(1-9) as raw materials, 0.5-1.0g of lipase Lipozyme RMIM as a catalyst and a tertiary amyl alcohol-DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme RMIM into a reaction channel of a microfluidic channel reactor, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, wherein the acylation reaction temperature is controlled at 40-55 DEG C, and the acylation reaction time is 15-35 minutes; and collecting the reaction solution on line, and carrying out conventional after-treatment on the reaction solution to obtain the 6''-O-palmitoyl-naringin ester. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
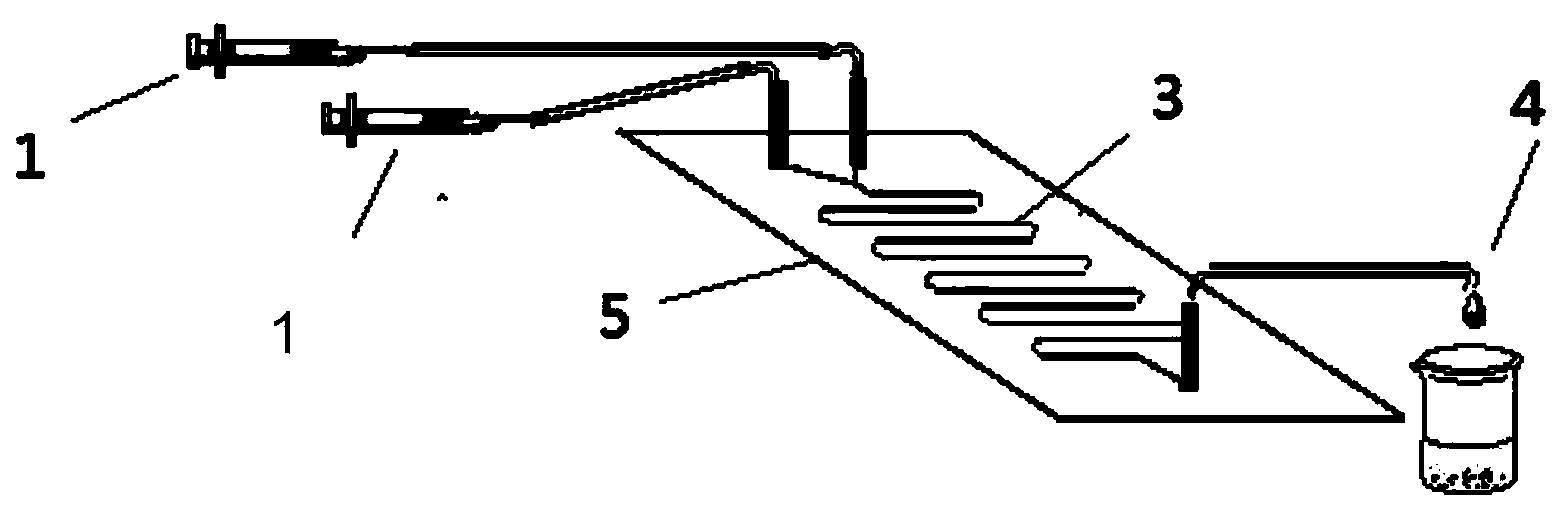
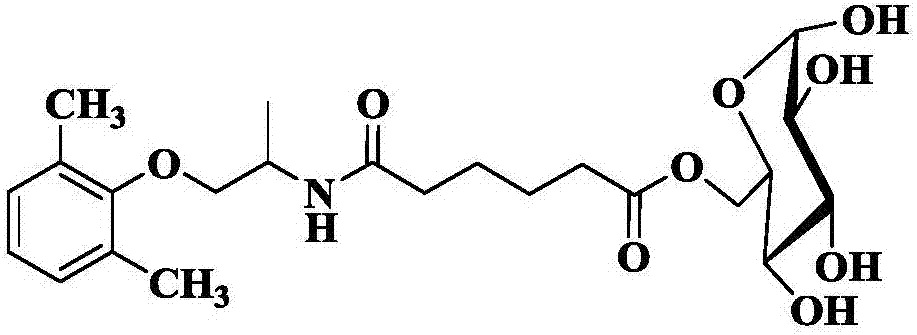
Method for lipase-catalyzed online synthesis of N-(5-glucose ester valeryl)mexiletine
InactiveCN107488690AShort reaction timeImprove conversion rateBioreactor/fermenter combinationsBiological substance pretreatmentsVinyl esterGlucose polymers
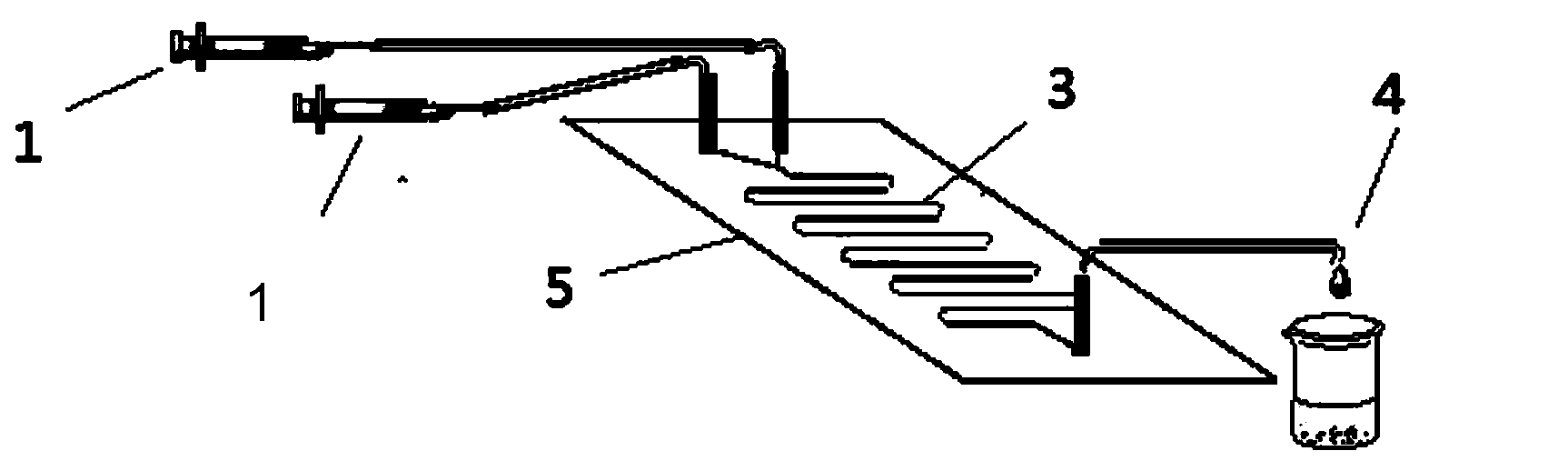
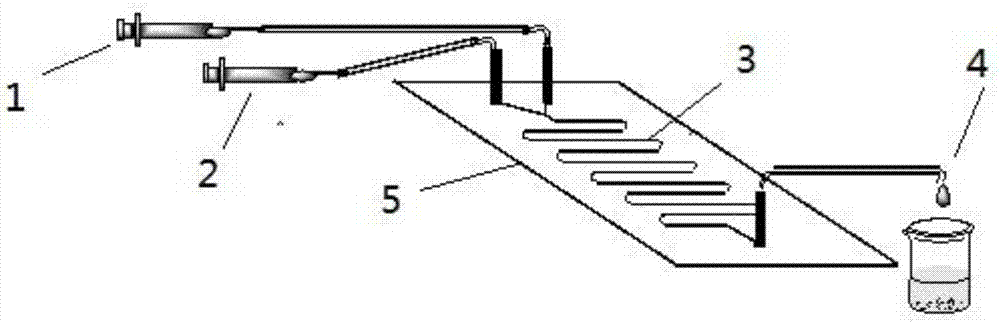
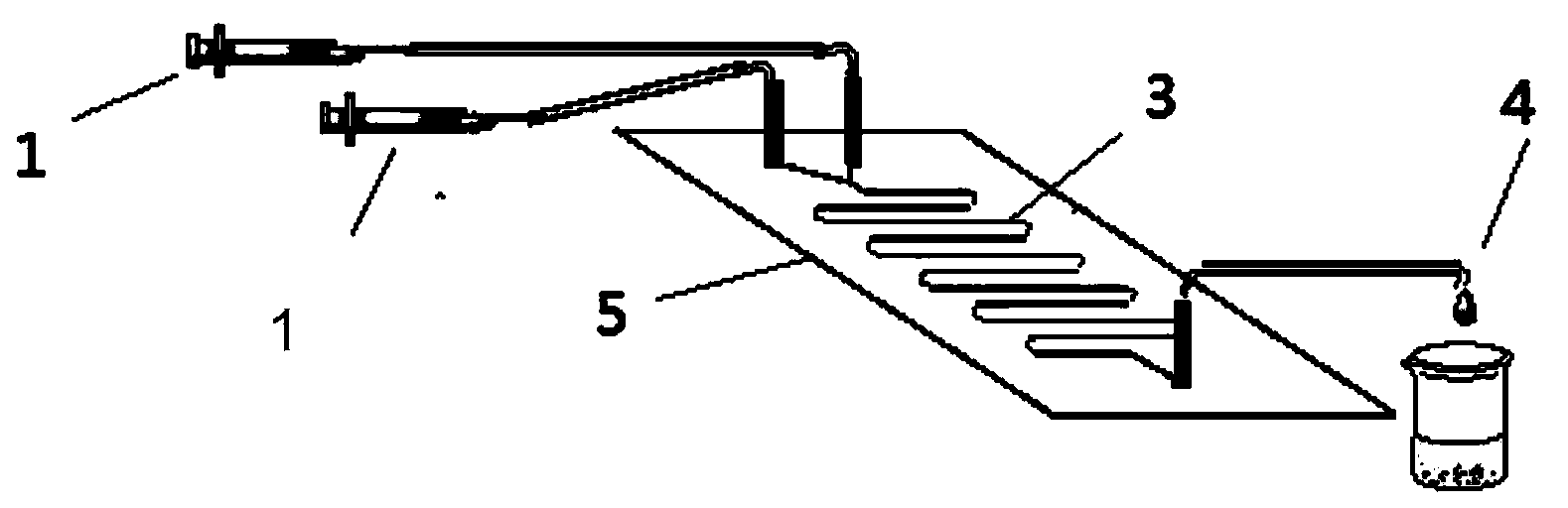
The invention discloses a method for lipase-catalyzed online synthesis of N-(5-glucose ester valeryl)mexiletine. The method, adopting N-(5-vinyl ester valeryl)mexiletine and glucose with a molar ratio of 1:(1-10) as raw materials, dimethyl sulphoxide and tertiary amyl alcohol as a reaction solvent and lipase Lipozyme TL IM as a catalyst, comprises the following steps: placing the raw materials and the reaction solvent in a syringe, uniformly filling the reaction channel of a micro-fluidic channel reactor with the lipase Lipozyme TL IM, and continuously introducing the raw materials and the reaction solvent into the reaction channel reactor under the push of a syringe pump, wherein the internal diameter of the reaction channel of the micro-fluidic channel reactor is 0.8-2.4 mm, and the length of the reaction channel is 0.5-1.0 m; and carrying out an esterification reaction at a temperature of 20-60 DEG C for 20-40 min, online collecting the obtained reaction solution through a product collector, and routinely post-processing the reaction to obtain the N-(5-glucose ester valeryl)mexiletine. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing 6''-O-lauroyl-neohesperidin dihydrochalcone ester on line by using lipase as catalyst
ActiveCN103667394AReduce usageShort reaction timeFermentationReaction temperatureMicrofluidic channel
The invention discloses a method for synthesizing a 6''-O-lauroyl-neohesperidin dihydrochalcone ester on line by using lipase as a catalyst, which comprises the following steps: by using neohesperidin dihydrochalcone and vinyl laurate in a mole ratio of 1:(1-12) as raw materials, 0.5-1.0g of lipase Lipozyme RMIM as a catalyst and a tertiary amyl alcohol-DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme RMIM into a reaction channel of a microfluidic channel reactor, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, wherein the acylation reaction temperature is controlled at 40-55 DEG C, and the acylation reaction time is 20-40 minutes; and collecting the reaction solution on line, and carrying out conventional after-treatment on the reaction solution to obtain the 6''-O-lauroyl-neohesperidin dihydrochalcone ester. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Preparation of 1,8-diazabicyclo[5.4.0]undec-7-ene
InactiveCN101279973AImprove conversion rateLow costOrganic chemistryHydrogenation reactionAcrylonitrile
The invention provides a method to prepare 1,8-dinitrogen-hexagon[5,4,0]hendecene(DBU) with caprolactam and acrylonitrile as material through addition reaction, hydrogenation reaction and cyclization reaction. The method is characterized in that tert-butyl alcohol or tertiary amyl alcohol is used as solvent and NaOH is used as catalyzer during the addition reaction which begins at the temperature of 10-15 DEG C; the hydrogenation reaction is carried out directly after the addition reaction, without recycling the solvent. The method has the advantages that (1) conversion rate of the materials is high, increased from the original 96% to 98%, and the yield of DBU is increased from 74% to above 80%, thus greatly reducing cost on the materials; (2) the process after the addition reaction is simplified and since the conversion rate of caprolactam is improved, the solvent and the caprolactam are of no need to recycle;(3) cost and consumption of catalyzer are reduced; (4)the same solvent is used in the addition reaction and the hydrogenation reaction, thus reducing the loss of solvent.
Owner:山东新华万博化工有限公司
Enzyme process acylation method of strawberry anthocyanin
The invention provides an enzyme process acylation method of strawberry anthocyanin, and belongs to the technical filed of deep processing of agricultural products. The method comprises the following steps: extracting strawberry anthocyanin by using edible alcohol, adding the above obtained crude extract liquid into an AB-8 macroporous resin column, preliminarily purifying, extracting fat-soluble impurities by using ethyl acetate, immediately adding the obtained liquid to a polyamide resin column, flushing the polyamide resin column with deionized water, eluting by using an aqueous solution of 30% ethanol, carrying out reduced pressure evaporation on the obtained eluate, dissolving the obtained material into tertiary amyl alcohol, adding cinnamic acid, Novozmy immobilized lipase and 4A molecular sieve, and carrying out a shocking reaction at 40DEG C for 72h, and filtering to remove the above enzyme and the molecular sieve. The method has the advantages of cycle use of the solvent and the enzyme, low cost, suitableness for mass production, and good industrial prospect. Acylated strawberry anthocyanin can be used as an anthocyanin theory research material, and can also be used in different fields as a natural food pigment.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method for synthesizing 6''-O-palmitoyl-neohesperidin ester on line by using lipase as catalyst
The invention discloses a method for synthesizing a 6''-O-palmitoyl-neohesperidin ester on line by using lipase as a catalyst, which comprises the following steps: by using neohesperidin and vinyl palmitate in a mole ratio of 1:(1-12) as raw materials, 0.5-1.0g of lipase Lipozyme RMIM as a catalyst and a tertiary amyl alcohol-DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme RMIM into a reaction channel of a microfluidic channel reactor, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, wherein the acylation reaction temperature is controlled at 40-55 DEG C, and the acylation reaction time is 20-40 minutes; and collecting the reaction solution on line, and carrying out conventional after-treatment on the reaction solution to obtain the 6''-O-palmitoyl-neohesperidin ester. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing tert-amyl alcohol
InactiveCN107879894AImprove the mixing effectIncrease mass transfer rateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydration reactionReaction rate
The invention discloses a method for preparing tert-amyl alcohol. The method disclosed by the invention comprises the following steps: enabling isopentene-enriched materials, water and promoter mixtures to simultaneously enter a static mixer, mixing, and introducing the mixed materials to enter a fixed bed reactor filled with a strong acid cation exchange resin to carry out hydration reaction; andsimply distilling and rectifying after the reaction is ended, thereby obtaining the high-purity product (tert-amyl alcohol) on a column reactor. According to the method disclosed by the invention, tetrabutylammonium bromide is added into a hydrate system to serve as the promoter, the contact environment between isopentene and an active center on the catalyst surface is improved, the liquid film lipophilicity on the catalyst surface is improved, contact resistance between isopentene and the catalyst surface is reduced, diffusion of isopentene to the active center of the catalyst surface is facilitated, and the reaction rate is further improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Quantitative detection method of aroma components in yellow serofluid
The invention discloses a quantitative detection method of aroma components in yellow serofluid, comprising pretreatment and unit detection processes. During the pretreatment process, an ethanol solution with its volume concentration being not less than 95% and yellow serofluid are uniformly mixed according to the volume ratio of 1:1 to obtain a mixed liquor; the mixed liquor is sealed and stands for 20-40 min, and then qualitative filter paper is used for coarse filtration and a coarse filtrate is collected or centrifugation is conducted and a supernatant is collected; the coarse filtrate or the supernatant is filtered by the use of a microporous membrane of 0.45 microns and the filtrate is collected to obtain a pretreatment fluid. According to the detection process, a mixed internal standard of 0.1 mL is added into the pretreatment fluid of 10 mL, and gas chromatography detection is carried out after uniform mixing, wherein the mixed internal standard is a mixture of tertiary amyl alcohol, n-amyl acetate and 2-ethylbutanoic acid which are mixed according to the volume ratio of 1: 1: 1. The technical scheme of the present invention is simple and rapid. In addition, by the adoption of the method, service safety of a gas chromatographic column can be effectively protected, and the service life of the gas chromatographic column is prolonged.
Owner:安徽润安信科检测科技有限公司
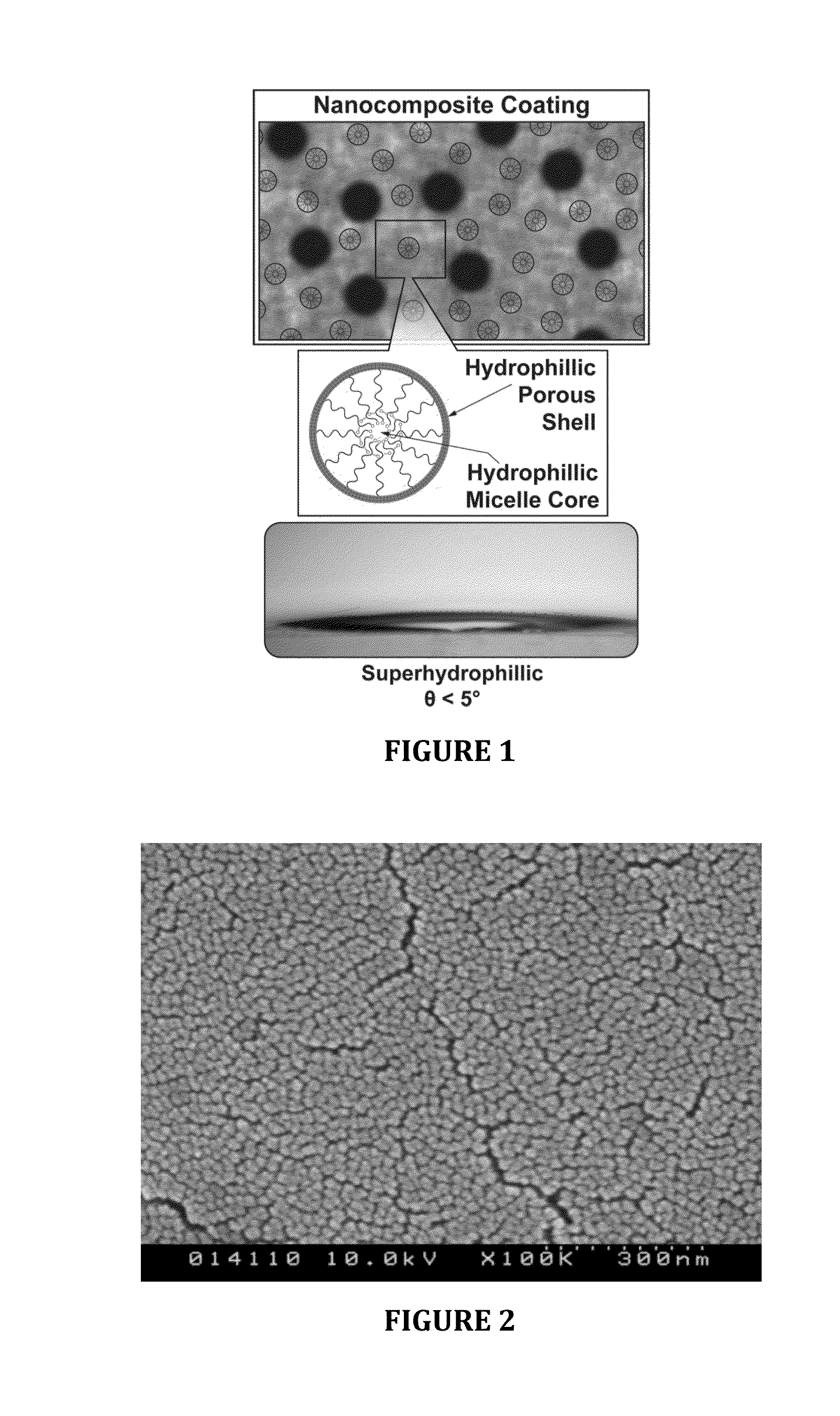
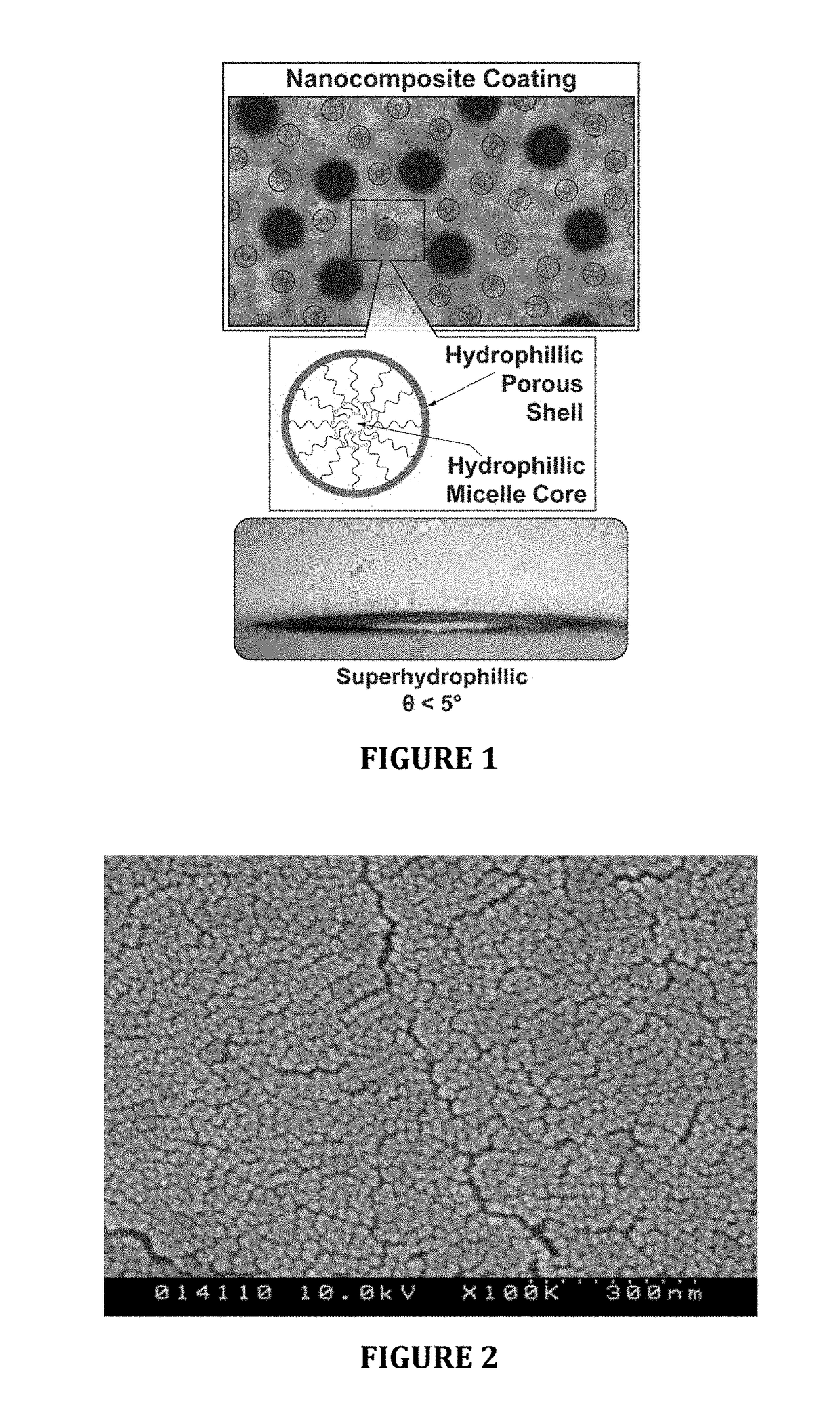
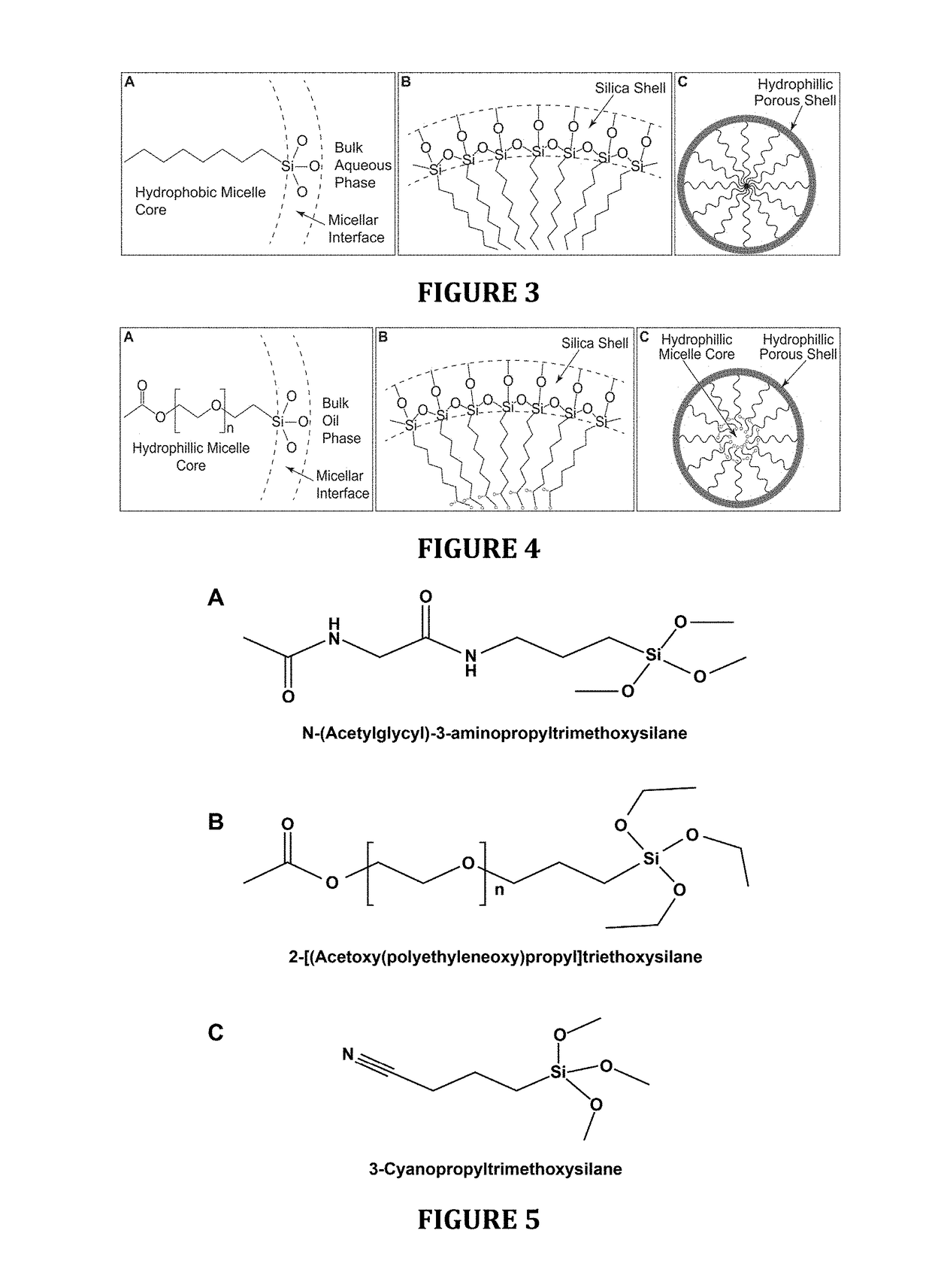
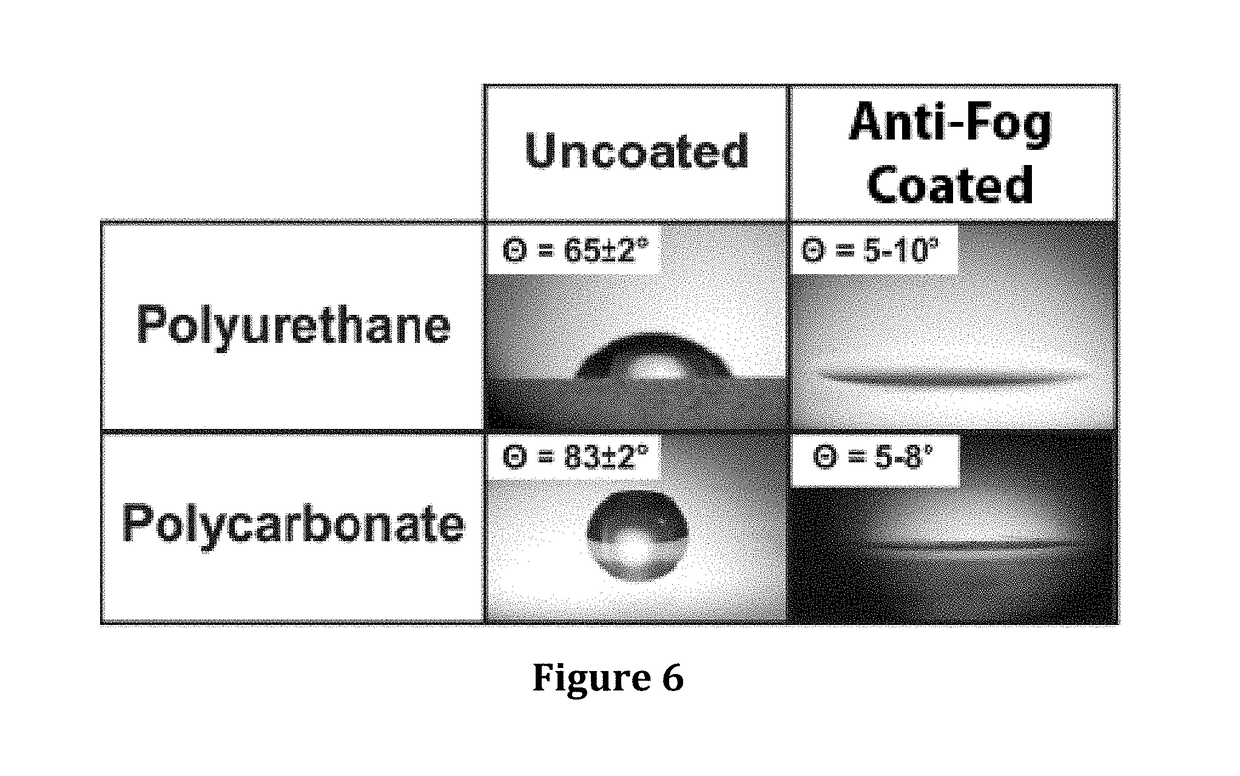
Hydrophilic anti-fog coatings
ActiveUS9840639B2Increased durabilityIncrease resistanceNanoopticsPolyurea/polyurethane coatingsColloidal silicaDiacetone alcohol
The present invention relates to hydrophilic anti-fog coatings. In particular, the coatings use two types of nanoscale particles, colloidal silica and porous organosilicate micelles, in a polyurethane matrix. The invention is an anti-fog coating for optically clear substrates (polycarbonate, polyurethane, nylon, polyester and other clear plastics) without the need for a primer and glass or oxide substrates with an additional primer layer, comprising monosized colloidal silica nanoparticles and porous organosilicate micelles in a polyurethane matrix. The silica is preferably 1-5% by weight and the micelles are loaded at 0.1 to 10% volume percentage by volume. The polyurethane prepolymer is dissolved at 10-40% by weight in a mixture of tertiary amyl alcohol and diacetone alcohol to customize for dip, flow or spray coating processes
Owner:INNOSENSE
Production process of tertiary amyl benzene
InactiveCN101607863AReduce dosagePhysical/chemical process catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationBenzeneAlkyl transfer
The invention discloses a production process of tertiary amyl benzene which is prepared by F-C alkylation of benzene and tertiary amyl alcohol. The production process comprises the following steps of: adding 6.53mol of benzene, 0.15mol of AlCl3 and 0.38mol of FeCl3 in a four-opening bottle, reducing the temperature to 0 to 5 DEG C, then adding 1mol of tertiary amyl alcohol by dropping for 1 hour, continuing reaction for 6 hours at the temperature of 0 to 5 DEG C after the dropwise adding, adding water for hydrolysis, standing and layering, separating the organic horizon, washing with water again, layering, absorbing water with calcium chloride, debenzolizing and conducting pressure reduction and distillation, thus obtaining the tertiary amyl benzene. In the production process, lewis acid AlCl3 and FeCl3 are used as catalysts, and the use amount of AlCl3 is reduced properly, thus avoiding the defects caused by only adopting AlCl3 or adopting two acids of AlCl3 and H2SO4 as the catalysts, leading the yield of tertiary amyl benzene to reach 90 percent and the content of isomer to be less than 0.2 percent.
Owner:葛秀龙
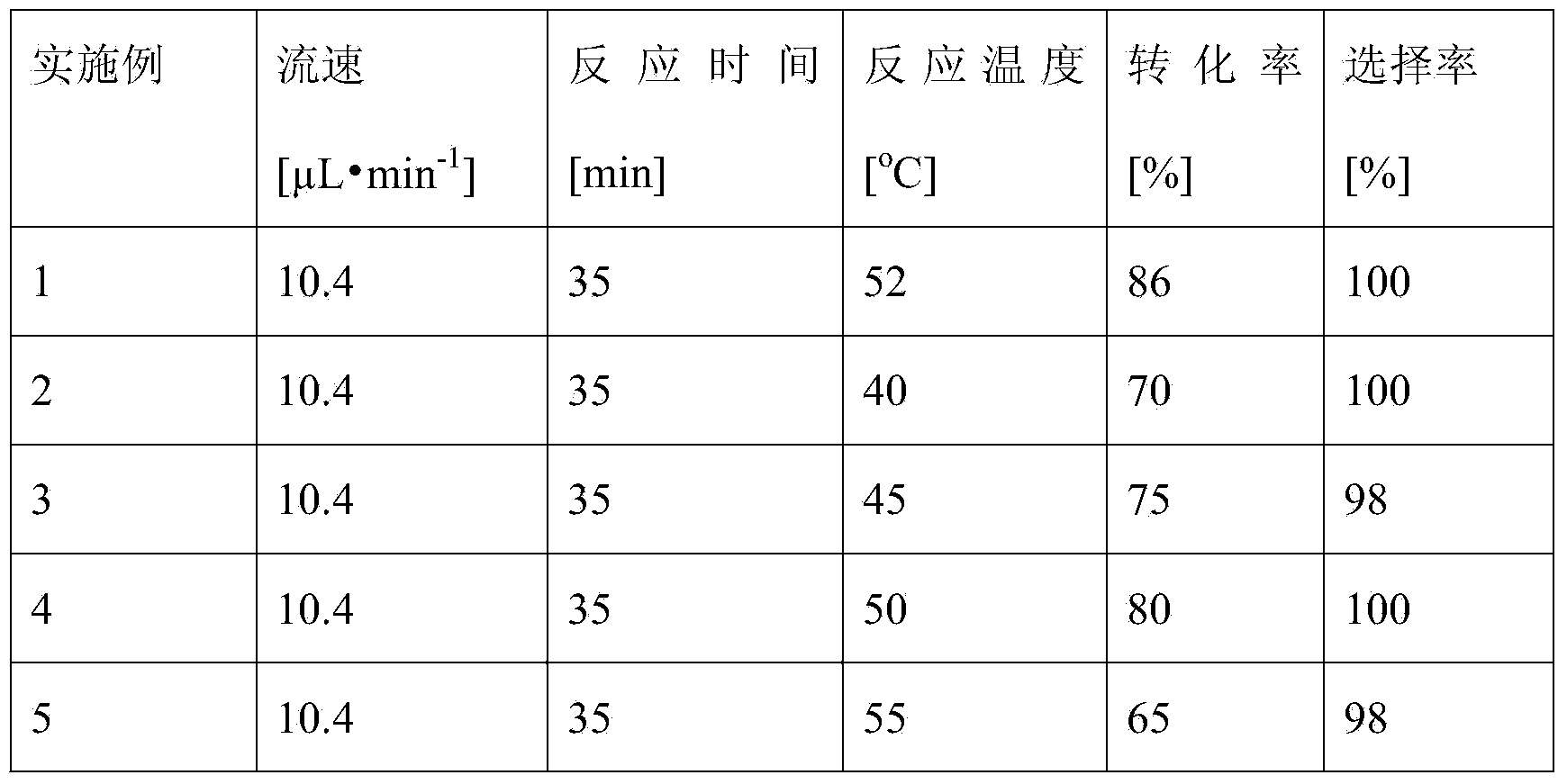
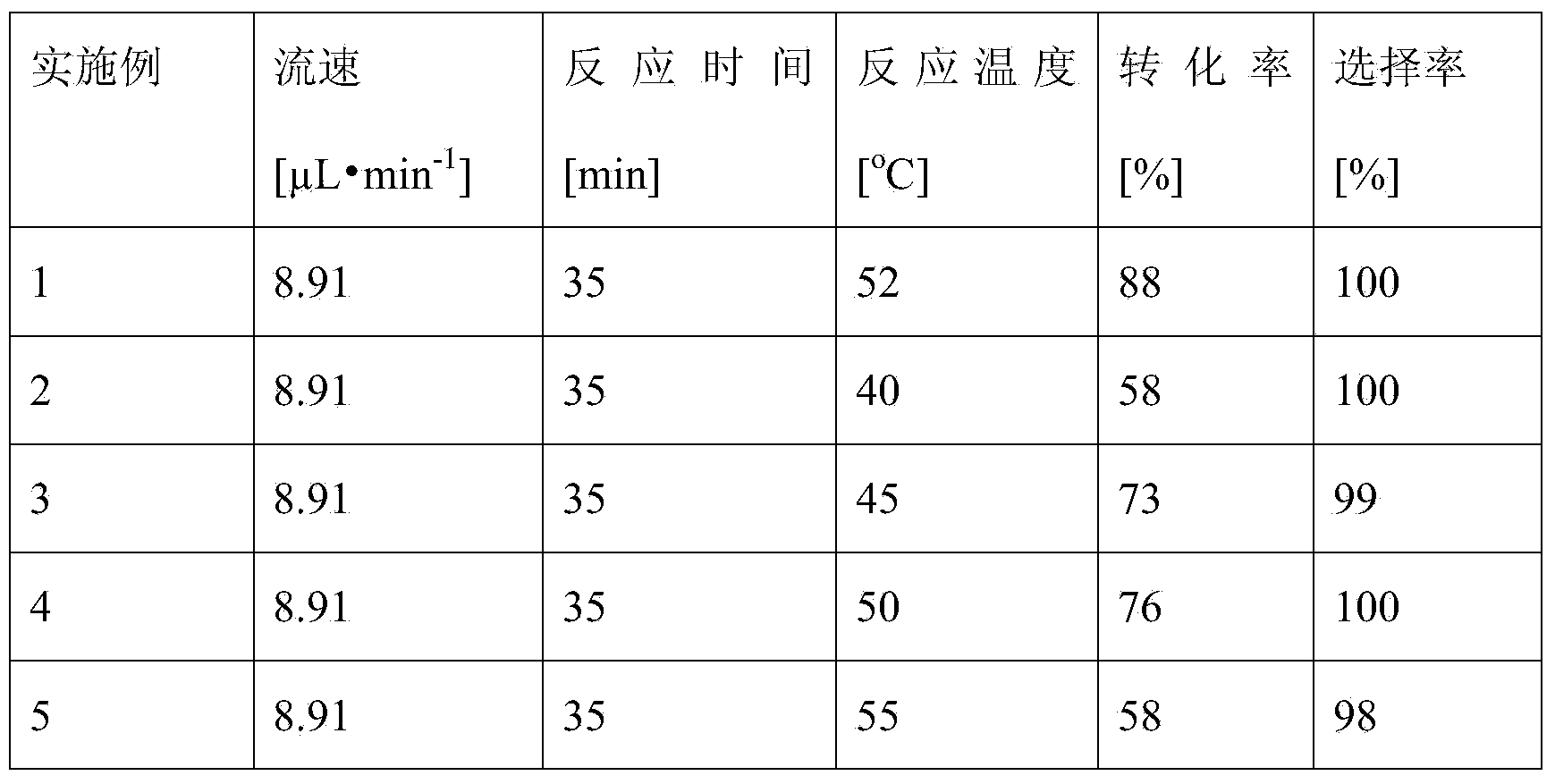
Method for on-line synthesizing mannose-6-acetate by lipase catalysis
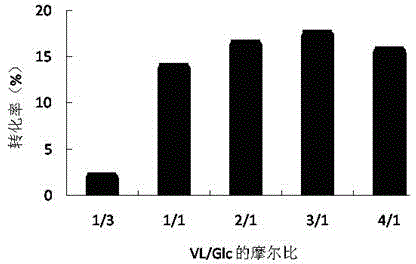
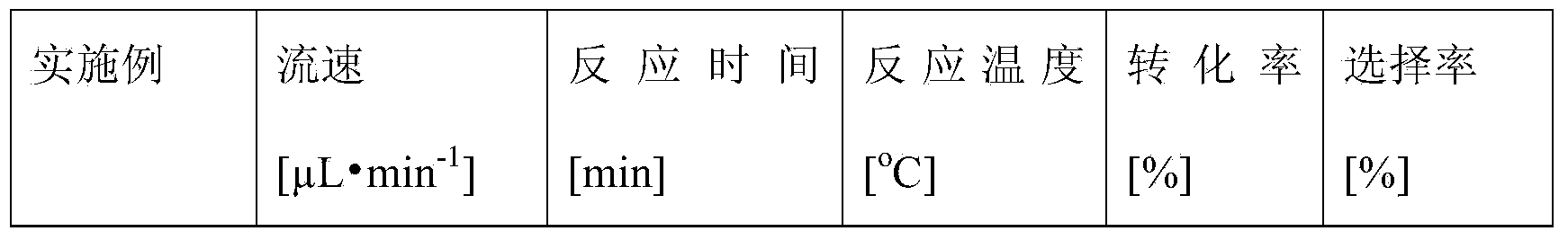
ActiveCN103184250AReduce usageShort reaction timeFermentationMicrofluidic channelTertiary amyl alcohol
Owner:ZHEJIANG UNIV OF TECH
Preparation of 1,8-diazabicyclo[5.4.0] hendecene
InactiveCN101279973BImprove conversion rateLow costOrganic chemistryHydrogenation reactionAcrylonitrile
Owner:山东新华万博化工有限公司
Method for synthesizing citric acid glyceride by immobilized lipase catalysis in multistage fixed bed reactor
A method for synthesizing citric acid glyceride by immobilized lipase catalysis in a multistage fixed bed reactor is disclosed, belonging to the technical field of the synthesis of citric acid glyceride. In the invention, monoglyceride and citric acid are used as reaction substrates, organic solvents like anhydrous isopropanol, anhydrous isoamylol or anhydrous tertiary amyl alcohol are used as reaction media, the immobilized lipase is used as a catalyst, and esterification reaction is carried out in the multistage fixed bed reactor to obtain the product of the citric acid glyceride. The invention, which takes the multistage fixed bed as the reactor and is provided with a molecular sieve packed column at the back of the reactors at every stage, facilitates the substrates to contact with the catalyst sufficiently, and meanwhile, removes the water generated from the reaction, in addition, the molecular sieve packed column is regenerated and refreshed periodically which is advantageous for the esterification reaction. Prohibition effect against the esterification reaction can be reduced by the addition of the citric acid and conversion rate is improved. The inventive method has the characteristics of mild condition, strong specificity, few byproducts, high conversion rate and the like.
Owner:JIANGNAN UNIV
Conversion process of primary alcohol, hexamethylene glycol, tertiary amyl alcohol or cyclohexanol into halohydrocarbon in acid ionic liquid [Hmim] X,X==Cl,Br or I)
InactiveCN1225440CSimple processRealize cleaner productionHalogenated hydrocarbon preparationHalohydrocarbonChemical reaction
The present invention relates to the conversion process of primary alcohol or cyclohexanol in methyl imidazole salt halide and belongs to the field of organic chemical reaction method. The ionic liquid is made to mix with primary alcohol or cyclohexanol through stirring, and the mixture is heated, let stand, gravitationally deposited and separated to obtain halohydrocarbon with the yield being 85-100%. In the reaction, the ionic liquid is used also as catalyst, halogenation reagent and solvent. The present invention has the advantages of high yield, simple technological process, circular use of catalyst and solvent, being environment friendly, etc.
Owner:EAST CHINA NORMAL UNIV
Hydrophilic Anti-fog coatings
ActiveUS20150344725A1Good anti-fog durabilityGood chemical resistanceOther chemical processesNanoopticsDiacetone alcoholColloidal silica
The present invention relates to hydrophilic anti-fog coatings. In particular, the coatings use two types of nanoscale particles, colloidal silica and porous organosilicate micelles, in a polyurethane matrix. The invention is an anti-fog coating for optically clear substrates (polycarbonate, polyurethane, nylon, polyester and other clear plastics) without the need for a primer and glass or oxide substrates with an additional primer layer, comprising monosized colloidal silica nanoparticles and porous organosilicate micelles in a polyurethane matrix. The silica is preferably 1-5% by weight and the micelles are loaded at 0.1 to 10% volume percentage by volume. The polyurethane prepolymer is dissolved at 10-40% by weight in a mixture of tertiary amyl alcohol and diacetone alcohol to customize for dip, flow or spray coating processes
Owner:INNOSENSE
Method for isomerizing 2-methyl-1-butylene into 2-methyl-2-butylene
InactiveCN101597201AImprove conversion rateHigh selectivityHydrocarbon by isomerisationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationFixed bed
The invention relates to a method for isomerizing 2-methyl-1-butylene into 2-methyl-2-butylene, which is used for improving the content of the 2-methyl-2-butylene in coarse isoamylene. The method comprises the following steps: tertiary amyl alcohol is added to the coarse isoamylene; and then the coarse isoamylene with the tertiary amyl alcohol is subjected to an isomerization reaction through a fixed bed catalyst bed layer consisting of sulfonic acid group cation exchange resin so as to ensure that the 2-methyl-1-butylene in the coarse isoamylene is converted into the 2-methyl-2-butylene, wherein the addition of the tertiary amyl alcohol takes the coarse isoamylene as a reference and is 0.1-2.0 percent by weight; the mass exchange capacity of the sulfonic acid group cation exchange resin is 3-5.5 mmol / g, and the mass space velocity is 1-35hr; the reaction temperature is 18-45 DEG C; and the reaction pressire is 0.3-1.0 MPa. The method effectively restrains respective or mutual dipolymer reactions of the 2-methyl-2-butylene and the 2-methyl-1-butylene in the process of the isomerization reaction.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for synthesizing citric acid glyceride by immobilized lipase catalysis in rotatory evaporator
A method for synthesizing citric acid glyceride by immobilized lipase catalysis in a rotatory evaporator is disclosed, belonging to the technical field of the synthesis of citric acid glyceride. In the invention, monoglyceride and citric acid are used as reaction substrates, organic solvents like anhydrous isopropanol, anhydrous isoamylol or anhydrous tertiary amyl alcohol are used as reaction media, the immobilized lipase is used as a catalyst, and esterification reaction is carried out in the rotatory evaporator to obtain the product of the citric acid glyceride. The invention, which takes the rotatory evaporator as the reactor, can facilitate the substrates to contact with the catalyst sufficiently. The inventive method has the characteristics of mild conditions, strong specificity, less number of byproducts, high conversion rate and the like.
Owner:JIANGNAN UNIV
Method for preparing isoamylene from methyl tert-amyl ether
InactiveCN101597200AImprove conversion rateHigh selectivityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon from oxygen organic compoundsIsomerizationGas phase
The invention relates to a method for preparing isoamylene from methyl tert-amyl ether, which comprises the following steps: (1) carrying out ether analysis reaction to methyl tert-amyl ether through a catalyst bed layer in a gas phase at 130-300 DEG C; (2) removing methanol in a product of the ether analysis reaction so as to obtain a coarse isoamylene material; (3) adding tertiary amyl alcohol to the coarse isoamylene material, and then carrying out isomerization reaction through the catalyst bed layer in a liquid phase so as to ensure that 2-methyl-1-butylene in the coarse isoamylene is converted into 2-methyl-2-butylene, wherein the addition of the tertiary amyl alcohol takes the coarse isoamylene as a reference and is 0.1-2.0 percent by weight, the mass space velocity is 1-35 hr, the reaction temperature is 18-45 DEG C, and the reaction pressure is 0.3-1.0MPa. Sulfonic acid group cationic exchange resin is used as a catalyst, and the mass exchange capacity of the catalyst is 3-5.5 mmol / g. The method effectively restrains respective or mutual dimerization reaction of the 2-methyl-2-butylene and the 2-methyl-1-butylene in the process of the isomerization reaction.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
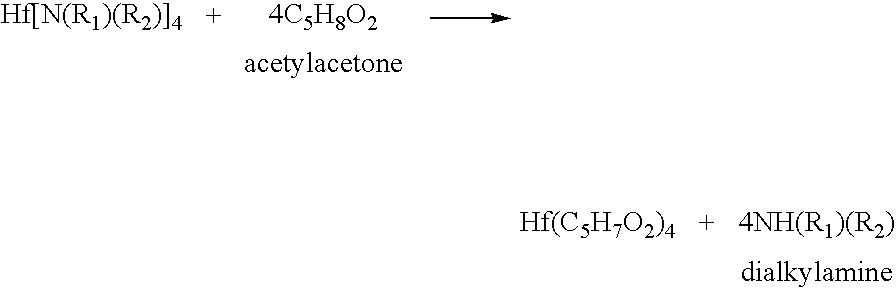
Processes for producing hafnium complexes
InactiveUS7518008B2High yieldEasily and safely removedOrganic compound preparationTitanium organic compoundsDistillationHafnium
Disclosed are first to sixth processes for respectively producing hafnium tetra-tertiary-butoxide, tetrakis(acetylacetonato)hafnium, tetrakis(1-methoxy-2-methyl-2-propanolato)hafnium, hafnium tetra-tertiary-amyloxide, tetrakis(3-methyl-3-pentoxy)hafnium, and tetrakis(hexafluoroacetylacetonato)hafnium. The first process includes the steps of (a) adding a compound A(OyXOnRf)m (e.g., CF3SO3H) to a crude hafnium amide Hf[N(R1)(R2)]4; (b) subjecting a product of the step (a) to a distillation under reduced pressure; (c) adding a lithium alkylamide Li(NR3R4) to a fraction obtained by the step (b); (d) subjecting a product of the step (c) to a distillation under reduced pressure; (e) adding tertiary butanol to a fraction obtained by the step (d); and (f) subjecting a product of the step (e) to a distillation under reduced pressure. The tertiary butanol of the step (e) is replaced with acetylacetone, 1-methoxy-2-methyl-2-propanol, tertiary amyl alcohol, 3-methyl-3-pentanol, and hexafluoroacetylacetone in the second to six processes, respectively.
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Conversion process of primary alcohol, hexamethylene glycol, tertiary amyl alcohol or cyclohexanol into halohydrocarbon in acid ionic liquid [Hmim] X,X==Cl,Br or I) Conversion process of primary alcohol, hexamethylene glycol, tertiary amyl alcohol or cyclohexanol into halohydrocarbon in acid ionic liquid [Hmim] X,X==Cl,Br or I)](https://images-eureka.patsnap.com/patent_img/5db63985-3442-4a77-8fe3-e7f200262037/C0311613100041.PNG)