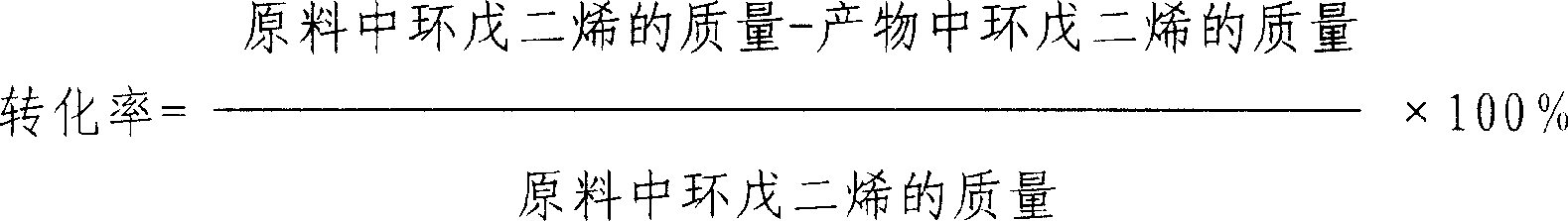
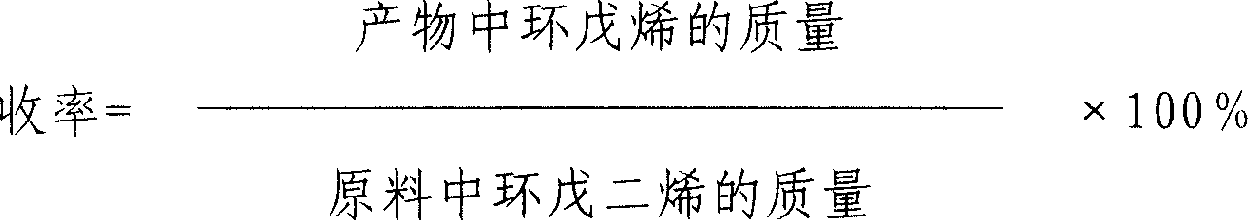
Method of preparing cyclopentene by continuous hydrogenation of cyclopentadiene
A technology for producing cyclopentadiene and cyclopentene, which is applied in the fields of hydrogenation, organic chemistry, etc., can solve the problems of reducing the yield of cyclopentene, limitation of cooling uniformity, and overheating of the catalyst bed, and achieves easy reaction , The effect of increasing the reaction yield and uniform temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
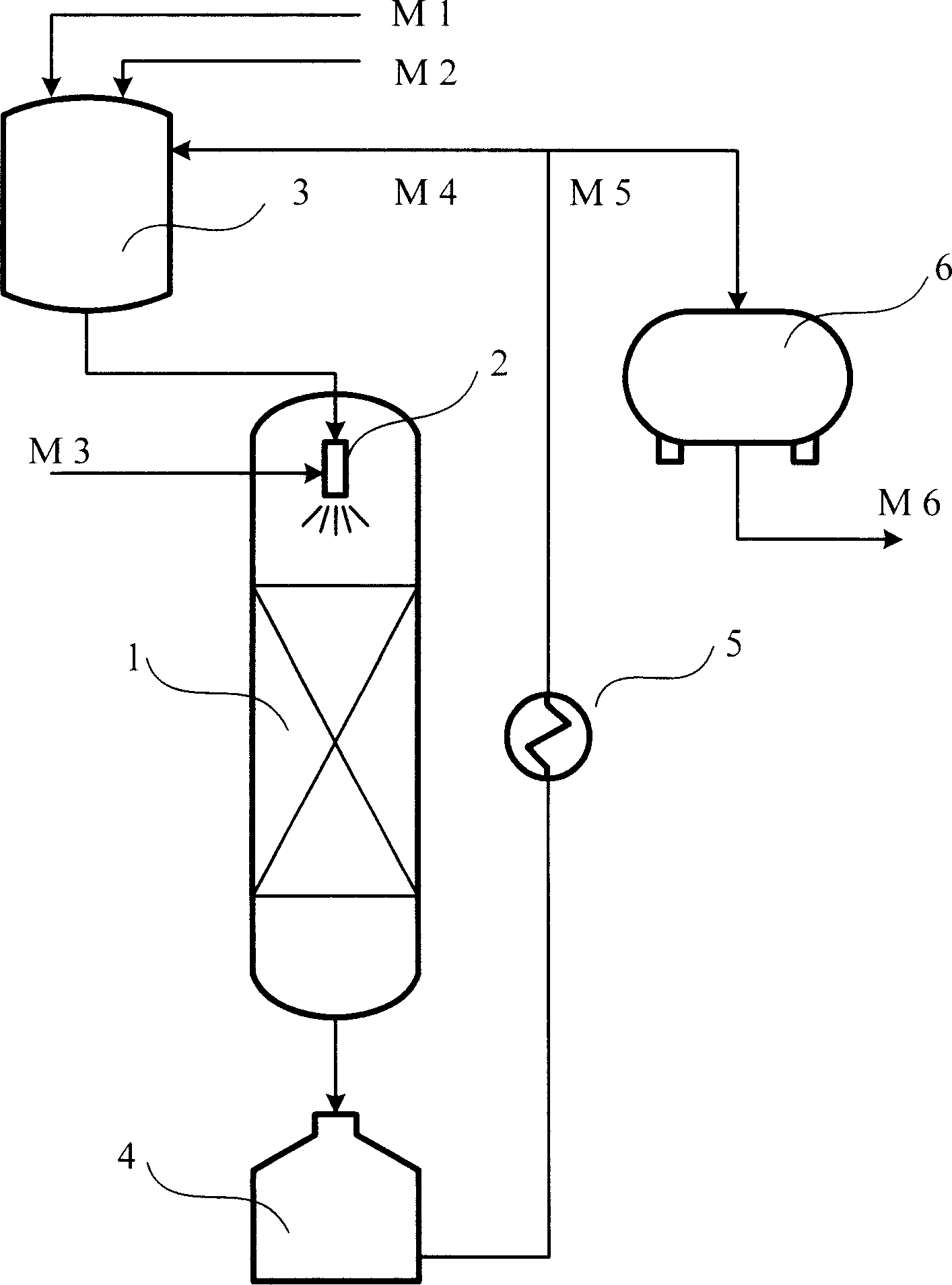
[0018] The process flow of each embodiment of the present invention is shown in the drawings. The reactor 1 is a fixed bed reactor, and a venturi ejector 2 is installed on the top of the reactor. The raw material cyclopentadiene M1, the solvent M2, and the external circulation reaction liquid M4 enter the raw material blending tank for mixing. The liquid phase material from the raw material blending tank and the raw material hydrogen M3 are fully mixed and atomized into a mist-like gas-liquid mixture in the Venturi injector 2 and then pass through the fixed-bed catalyst bed for hydrogenation reaction. The reaction liquid from the reactor enters the reaction liquid storage tank 4, and then passes through the external circulation heat exchanger for heat exchange and cooling. The cooled reaction liquid is divided into two parts, one is the external circulation part M4, the other is the discharge M5, M4 returns to the raw material blending tank 3, and M5 enters the product storage tan...
Embodiment 9
[0021] Except that methanol is used as the solvent, the rest are the same as in Examples 1-8, and other process conditions are listed in Table 1.
Embodiment 10
[0023] Except that tert-amyl alcohol is used as the solvent, the rest is the same as in Examples 1-8, and other process conditions are listed in Table 1.
[0024] The reaction results of each example are shown in Table 2.
[0025] CPD / solvent
(weight ratio)
CPD / H 2
(mol ratio)
Reaction pressure
(MPa)
temperature reflex
(℃)
Catalyst load
(hr -1 )
M5 / M4
(weight ratio)
Example 1
1∶5
1∶3.2
1.2
55
7.0
1∶10
Example 2
1∶8
1∶2.1
1.2
46
6.0
1∶5
Example 3
1∶8
1∶2.1
0.9
45
5.5
1∶8
Example 4
1∶8
1∶2.1
1.5
43
5.5
1∶8
Example 5
1∶8
1∶2.1
1.2
43
5.5
1∶8
Example 6
1∶8
1∶2.4
1.2
50
3.5
1∶12
Example 7
1∶8
1∶1.5
1.2
40
5.5
1∶4
Example 8
1∶10
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com