Method of preparing methyl cyclo penlene by continuous hydrogenation of methyl cyclo pentadiene
A technology for producing methylcyclopentadiene and methylcyclopentene, which is applied in hydrogenation to hydrocarbons, organic chemistry, etc., and can solve problems such as catalyst bed overheating, reduction in cyclopentene yield, and cooling uniformity limitations , to achieve the effect of easy reaction, high reaction yield and uniform temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
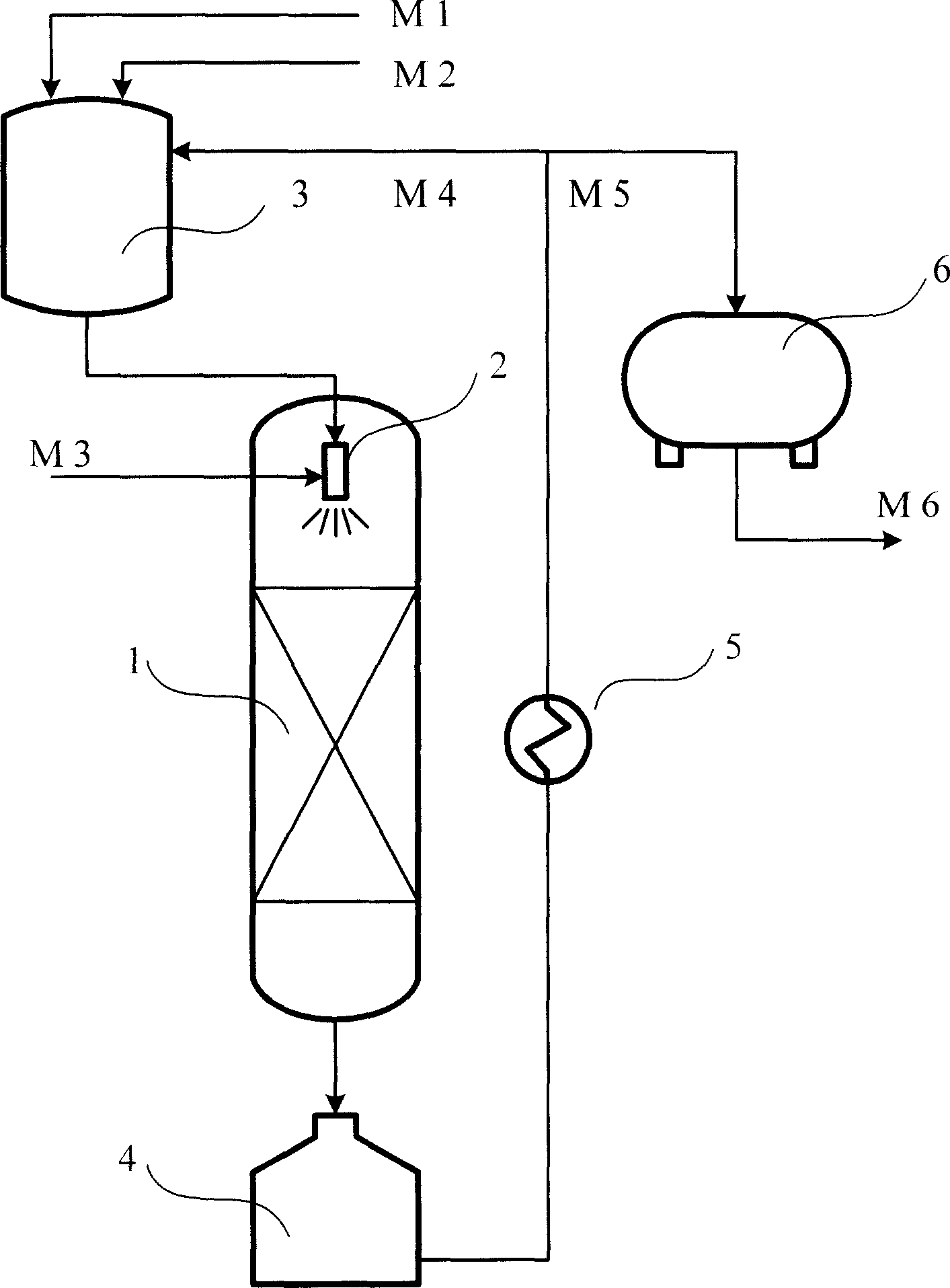
[0017] The process flow of each embodiment of the present invention is shown in the accompanying drawings. The reactor 1 is a fixed-bed reactor, and a Venturi injector 2 is arranged on the top of the reactor. The raw material methylcyclopentadiene M1, the solvent M2 and the externally circulated reaction solution M4 enter the raw material blending tank for mixing. The liquid phase material from the raw material preparation tank and the raw material hydrogen M3 are fully mixed and atomized in the Venturi injector 2 to form a mist-like gas-liquid mixture, and then pass through the fixed-bed catalyst bed for hydrogenation reaction. The reaction liquid from the reactor enters the reaction liquid storage tank 4, and then passes through the external circulation heat exchanger for heat exchange and cooling. The cooled reaction solution is divided into two parts, one is the external circulation part M4, the other is the discharge M5, M4 returns to the raw material preparation tank 3, ...
Embodiment 9
[0020] All the other are the same as in Examples 1-8 except that methanol is used as the solvent, and other process conditions are listed in Table 1.
Embodiment 10
[0022] Except that the solvent adopts tert-amyl alcohol, all the others are the same as in Examples 1-8, and other process conditions are listed in Table 1.
[0023] The reaction results of each embodiment are shown in Table 2.
[0024] MCPD / solvent
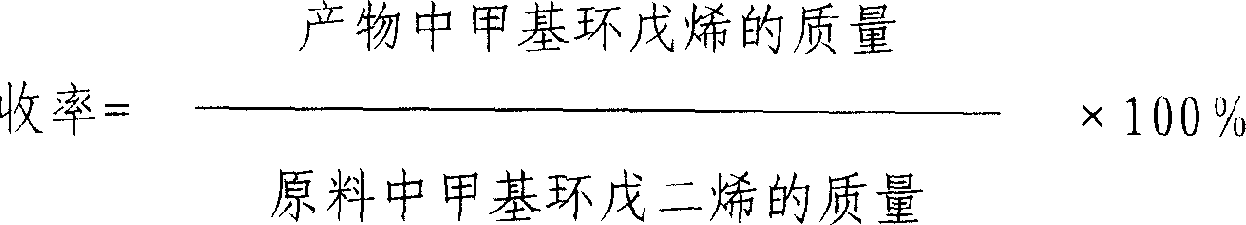
[0025] MCPD conversion rate
[0026] (Note: MCPD in the table is methylcyclopentadiene, M5 is the discharge amount of the reaction solution, and M4 is the external circulation amount of the reaction solution.)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com