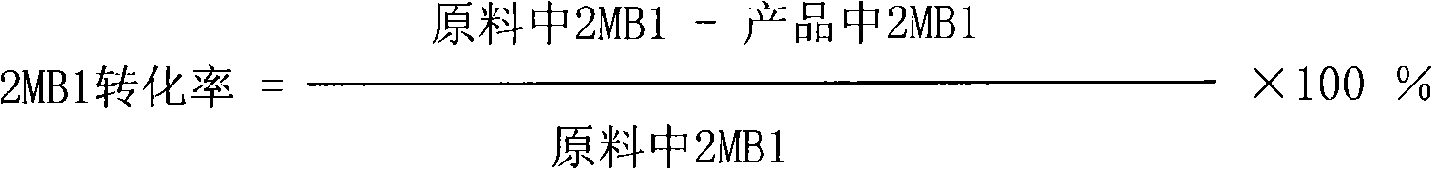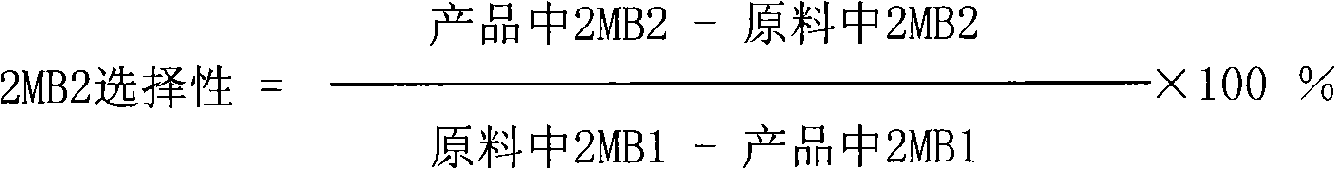Method for preparing isoamylene from methyl tert-amyl ether
A technology of methyl tert-amyl ether and isopentene, which is applied in chemical instruments and methods, hydrocarbon production from oxygen-containing organic compounds, catalysts for physical/chemical processes, etc., can solve the abnormal fluctuation of catalyst bed temperature and the increase of energy consumption , product yield reduction and other problems, to achieve the effect of eliminating abnormal fluctuations in bed temperature, reducing content, conversion rate and selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0023] 1. Thermal cracking of methyl tert-amyl ether
[0024] The reaction raw material is methyl tert-amyl ether obtained by reacting the carbon five fraction of the by-product of naphtha steam cracking to produce ethylene and methanol. After the methyl tert-amyl ether is heated and vaporized, it passes through the gas phase to modify α-Al by HF 2 O 3 The packed catalyst bed undergoes etherolysis reaction, and the reaction temperature is controlled at 180-230°C. The etherification reaction product water eluted methanol in it to obtain crude isoamylene. The components are shown in Table 1.
[0025] Table 1.
[0026] 2MB2
(wt%)
2MB1
(wt%)
2MB2 / 2MB1
(Quality ratio)
TAME
(wt%)
(wt%)
Other impurities such as carbon five
74.18
25.04
2.96
0.57
0.04
Margin
[0027] 2. Isomerization reaction
[0028] The isomerization reaction was carried out in a stainless steel tubular fixed-bed reactor w...
Embodiment 3
[0039] The isomerization reaction product obtained in Example 3 is further subjected to rectification and refining, and refined isoamylene is produced in the side stream, and light component and heavy component impurities are discharged from the top of the tower and the bottom of the tower, respectively. The specific distillation conditions are shown in Table 4, and the refined isoamylene product is analyzed by chromatographic analysis. The main indicators are shown in Table 5.
[0040] Table 4.
[0041] Tower kettle temperature
(℃)
Tower top temperature
(℃)
Operating pressure
(MPa)
Reflux ratio
Example 11
125
68
0.50
3.0
Example 12
120
63
0.12
2.0
Example 13
123
65
0.30
3.0
Example 14
115
59
0.10
1.0
[0042] table 5.
[0043] 2MB2 content
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com