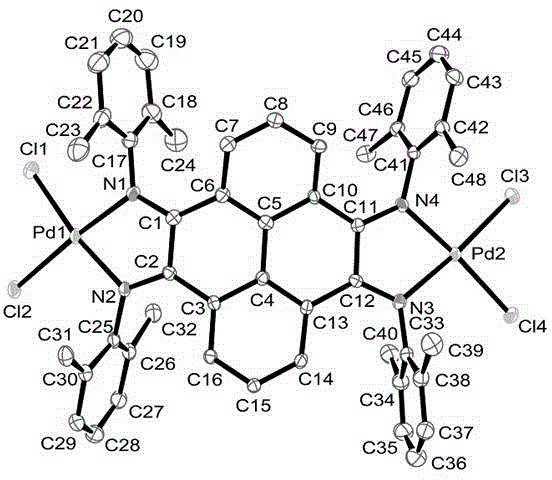
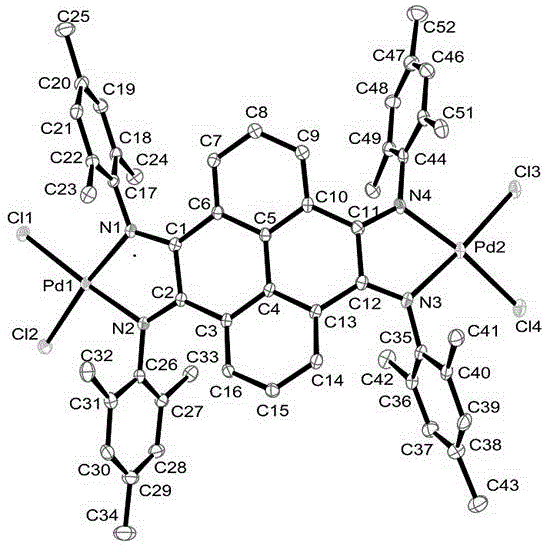
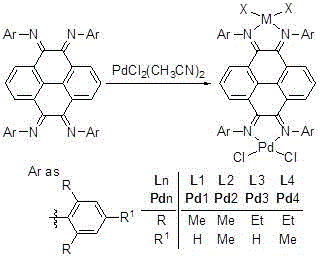
Pyrene-4,5,9,10-quadri-imine-(arylamine) palladium chloride and application thereof in Heck reaction
A reaction and catalyst technology, applied in the field of chemistry, can solve the problems that palladium black is difficult to recycle and reuse, palladium powder is difficult to separate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthetic method for preparing pyrene-4,5,9,10-tetraimine-(2,6-dimethylanilino)palladium(II) chloride [complex C1].
[0067]
[0068] 0.54 g (2.0 mmol) PdCl 2 (CH 3 EN) 2 Add 0.80 g (1.2 mmol) pyrene-4,5,9,10-tetraimine-(2,6-dimethylanilino) into a schlenk tube with 5 mL of dichloromethane, PdCl 2 (CH 3 EN) 2 The molar ratio to imine was 2.0:1.2, stirred at 30°C for 12 h, part of the dichloromethane was pumped out by a diaphragm pump, and 30 mL of diethyl ether was added, a reddish-brown solid precipitated, filtered through filter paper, and the filtrate was washed with diethyl ether (5 mL ×3), the washed solid was dried in a vacuum oven at 30 ℃ for 4 h to obtain 0.93 g of reddish-brown solid. Yield: 90.2%. 1 H NMR (400 MHz, CDCl 3 , ppm): 7.20 (d, J = 13.2 Hz, 4H, Ph- H ), 7.08 (d, J = 12.8 Hz, 6H, Ph- H ), 6.70-6.90 (m, 8H, Ph- H ), 2.32 (s, 6H, 2×C H 3 ), 2.18 (s, 6H, 2×C H 3 ), 1.91 (s, 6H, 2×C H 3 ), 1.32 (s, 6H, 2×C H 3 ).
[0069] 13 C...
Embodiment 2
[0071] Synthetic method for preparing pyrene-4,5,9,10-tetraimine-(2,6-diethylanilino)palladium(II) chloride [complex C2].
[0072]
[0073] 0.54 g (2.0 mmol) PdCl 2 (CH 3 EN)2 Add to 0.93 g (1.2 mmol) pyrene-4,5,9,10-tetraimine-(2,6-diethylanilino) into a schlenk tube with 5 mL of dichloromethane, PdCl 2 (CH 3 EN) 2 The molar ratio to imine was 2.0:1.2, stirred at 30°C for 12 h, part of the dichloromethane was pumped out by a diaphragm pump, and 30 mL of diethyl ether was added, a reddish-brown solid precipitated, filtered through filter paper, and the filtrate was washed with diethyl ether (5 mL ×3), the washed solid was dried in a vacuum oven at 30 ℃ for 4 h to obtain 0.68 g of reddish-brown solid. Yield: 59.6%.
Embodiment 3
[0075] Synthetic method for preparing pyrene-4,5,9,10-tetraimine-(2,4,6-trimethylanilino)palladium(II) chloride [complex C3].
[0076]
[0077] 0.54 g (2.0 mmol) PdCl 2 (CH 3 EN) 2 Add 0.87 g (1.2 mmol) pyrene-4,5,9,10-tetraimine-(2,4,6-trimethylanilino) to a schlenk tube with 5 mL of dichloromethane, PdCl 2 (CH 3 EN) 2 The molar ratio to imine was 2.0:1.2, stirred at 30°C for 12 h, part of the dichloromethane was pumped out by a diaphragm pump, and 30 mL of diethyl ether was added, a reddish-brown solid precipitated, filtered through filter paper, and the filtrate was washed with diethyl ether (5 mL ×3), the washed solid was dried in a vacuum oven at 30 °C for 4 h to obtain 0.90 g of reddish-brown solid. Yield: 83.3%.
[0078] 1 H NMR (400 MHz, CDCl 3 , ppm): 7.05 (s, 4H, Ph- H ), 6.97-7.01 (m, 4H, Ph- H ), 6.76-6.80 (m, 3H, Ph- H ), 6.53-6.69 (m, 3H, Ph- H ), 2.38 (s, 6H, 2×C H 3 ), 2.19-2.37 (m, 24H, 8×C H 3 ), 1.30 (s, 6H, 2×C H 3 ). 13 C NMR (100 MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com