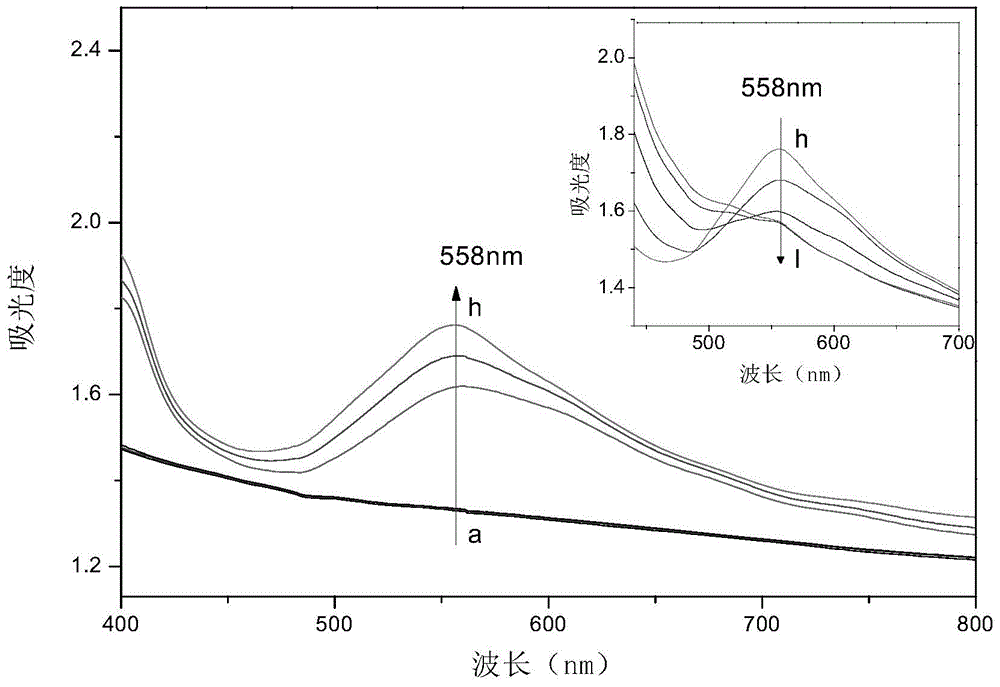
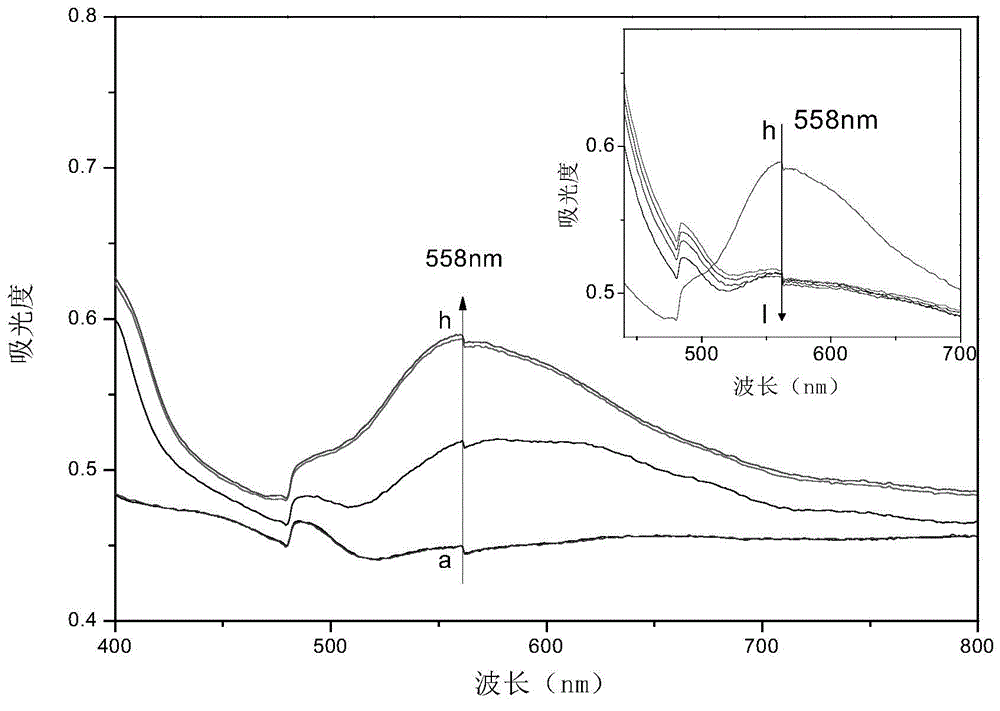
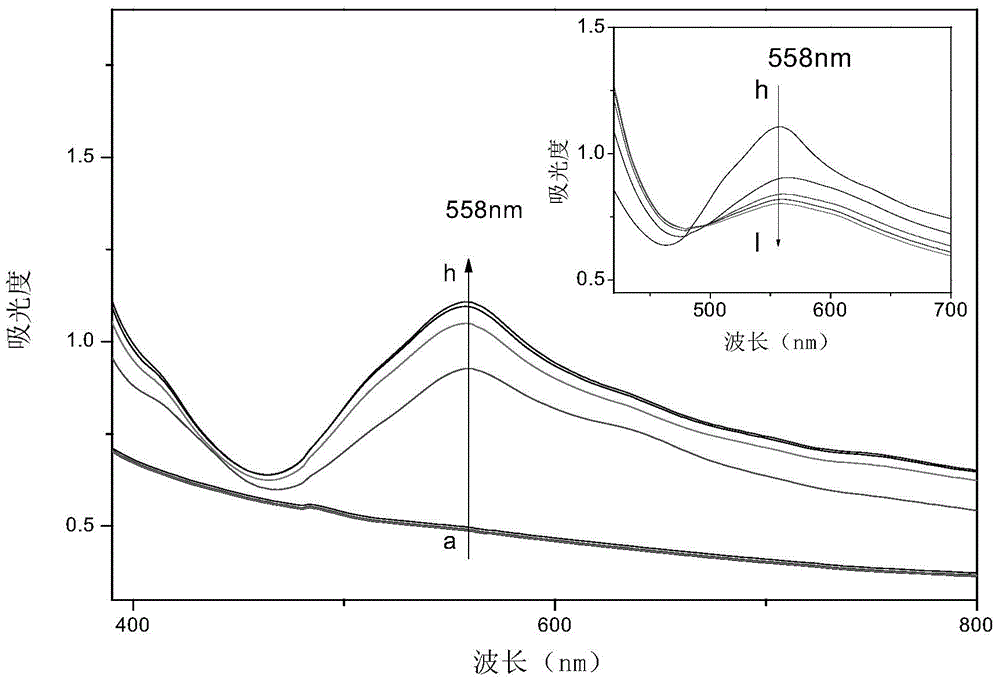
Viologen compound, and preparation method and application thereof
A technology of compound and alkyl, which is applied in the field of chemical industry and materials, and electrochromic materials, can solve the problems of weak electroactivity, large quality loss in the post-processing process, and high cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: 1,1'-bis(n-hexadecyl)-4,4'-bipyridinium dibromide (HV) preparation of
[0097]Dissolve 0.0025mol 4,4'-bipyridine in 5mL DMF (N'N dimethylformamide), then add 0.01mol hexadecane bromide solution diluted with 5mL DMF, stir magnetically for 30 minutes, and place Heat the reaction at a constant temperature of 60°C in an oil bath for 72h. Then, excess anhydrous diethyl ether was added to the reacted solution to form a precipitate, filtered by suction, and finally dried in a vacuum oven at 70°C to obtain a yellow powder. The dried powder was recrystallized, and the operation was repeated three times to obtain yellow crystals, that is, 1,1'-bis(n-hexadecyl)-4,4'-bipyridyl dibromide, with a yield of 76%. 1 H NMR (300MHz, MeOD): 0.92(t,6H); 1.25-1.51(m,52H); 2.12(d,4H); 4.78(t,4H); 8.70(d,4H); ).
[0098] The reaction formula is as follows:
[0099]
[0100] Wherein, the steps of recrystallization are as follows:
[0101] Put the dried powder into a 50mL r...
Embodiment 2
[0103] Example 2: 1,1'-bis(n-dodecyl)-4,4'-bipyridylium dibromide (DoV) preparation of
[0104] Dissolve 0.0025mol 4,4'-bipyridine in 5mL DMF, add 0.01mol bromododecane solution diluted with 5mL DMF, stir magnetically for 30 minutes, then place in an oil bath at a constant temperature of 60°C and heat for 72h . Then add excess anhydrous diethyl ether to the reacted solution to form a precipitate, filter it with suction, and finally put it in a vacuum oven at 70°C to dry to obtain a dark yellow powder; dissolve the dried powder into an acetonitrile solution for re- The crystallization operation was performed three times (the specific operation steps of recrystallization are exactly the same as in Example 1), and light yellow crystals were obtained, namely 1,1'-bis(n-dodecyl)-4,4'-bipyridyl dibromide, producing rate of 74%. 1 H NMR (300MHz, MeOD): 0.92(t,6H); 1.25-1.51(m,36H); 2.12(d,4H); 4.75(t,4H); 8.70(d,4H); ).
[0105] The reaction formula is as follows:
[0106] ...
Embodiment 3
[0107] Embodiment 3: Preparation of electrochromic thin film material 1
[0108] Take an appropriate amount of 1,1'-bis(n-hexadecyl)-4,4'-bipyridinium dibromide (HV) prepared in Example 1, and dissolve it in ethanol / water=1 / 1 (volume ratio) In the solvent, the HV solution of 1-10mmol / L is obtained after the dissolution is complete, and an appropriate amount of solution is dropped on the conductive surface of the ITO conductive glass. In order to ensure that the thickness of the prepared film is consistent, the HV solution dropped on the ITO is constant. After uniform scraping, put it into an oven with a temperature of 80°C for rapid drying and prepare it as an HV electrochromic film material coated on ITO conductive glass.
[0109] Thus, the electrochromic thin film material 1 was produced.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com