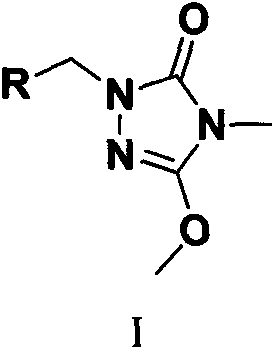
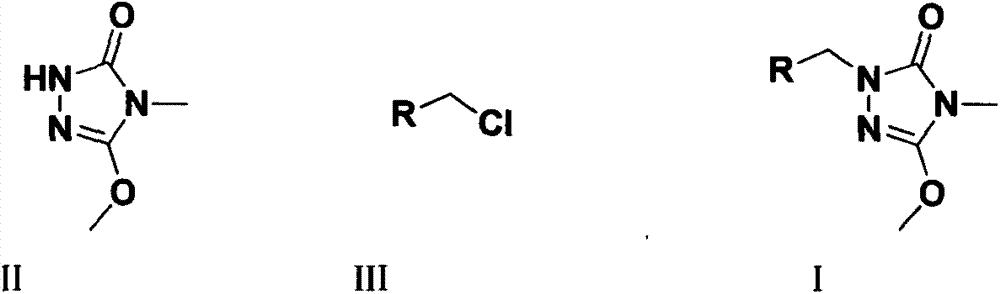
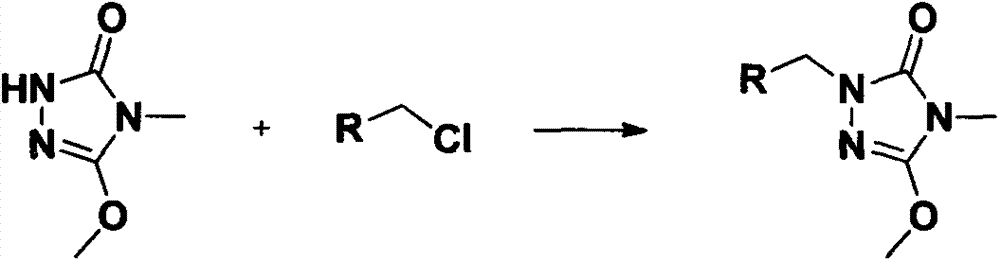
Preparation and application of a kind of triazolone compound
A kind of technology of triazolone and compound, applied in the field of triazolone compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The target compound is the synthesis of formula (I) compound: add 2mmol compound II, 2mmol substituted benzyl chloride or chloride, 8mL DMF and 2.4mmol K in a 25mL round bottom flask 2 CO 3 , stirred at room temperature, and TLC was used to detect the progress of the reaction. After the reaction was completed, the reaction solution was poured into 30 mL of ice water to precipitate a solid, which was filtered, precipitated, and subjected to silica gel column chromatography to obtain a solid product. The following specific compounds were all synthesized by the same method.
[0024] 1-(4-fluorophenyl)-3-methoxy-4-methyl-1H-1,2,4-triazol-5(4H)-one (2a)
[0025] m.p.102-103°C, Yield 91%. 1 H NMR (400M, CDCl 3 ): 3.15 (s, 3H, N-CH 3 ), 3.95(s, 3H, OCH 3 ), 4.97 (s, 2H, NCH 2 ), 7.05-7.12(m, 2H, Ph), 7.27-7.29(m, 2H, Ph); ESI-MS: 238[M+H].
[0026] 1-(4-Bromophenyl)-3-methoxy-4-methyl-1H-1,2,4-triazol-5(4H)-one (2b)
[0027] m.p.138-140°C, Yield 88%. 1 H NMR (400M, C...
Embodiment 2
[0039] Bactericidal activity test
[0040] 2.1 Test drug
[0041] 2.1.1 Test agents and treatment doses
[0042] For the test samples, each sample has a test concentration of 500mg / L.
[0043] 2.1.2 Tested strains
[0044] Cucumber scab: Cucumber brown spot: Cucumber powdery mildew; all preserved by Vegetable and Flower Institute, Chinese Academy of Agricultural Sciences.
[0045] 2.2 Cell Arrangement
[0046] All treatments were replicated 3 times, and 10 seedlings were replicated each time, and randomly arranged in the greenhouse.
[0047] 2.3 Test treatment
[0048] 2.3.1 Application and inoculation methods
[0049] In the morning of a sunny day, the test agent and the control agent were prepared according to the test concentration. The inoculation method of cucumber scab, cucumber brown spot, and powdery mildew was inoculated by spraying spore suspension, and moisturizing culture after inoculation.
[0050] 2.3.2 Meteorological data
[0051] During the test period, i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com