Method for rapidly preparing six compounds in Stellera chamaejasme L.
A technology of chamaejasma chamaejasme and compounds, which is applied in the field of rapid preparation of 6 compounds in chamaejasma chamaejasme, achieving the effects of high separation efficiency, high product purity and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
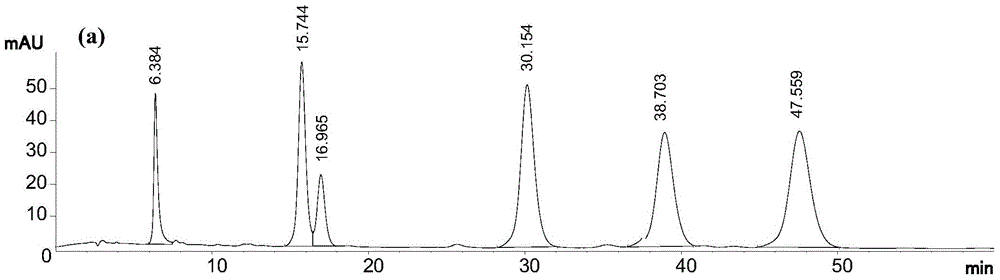
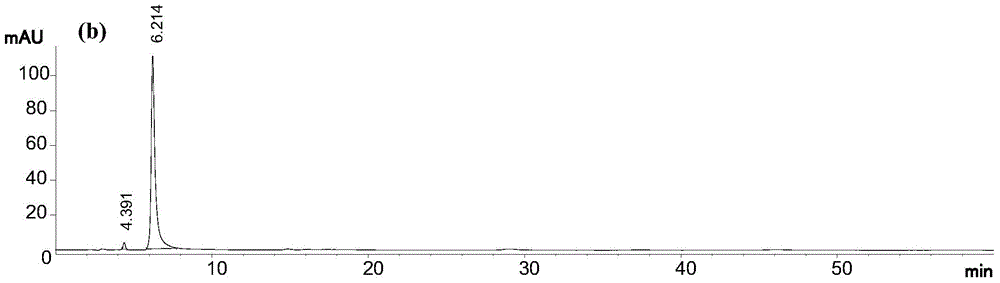
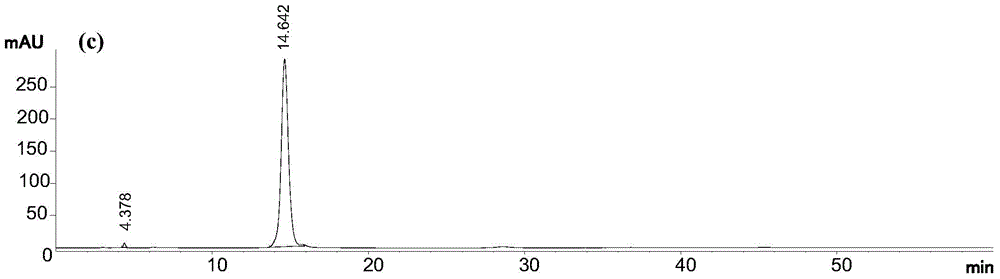
[0036] In this example, using 95% (volume concentration, the same below) ethanol extract of Daphne chamaejasme as raw material, the first step of normal phase column chromatography uses 200-300 mesh silica gel, and the second step of reverse phase column chromatography uses 40 ~50 μm ODS, the third step of HPLC preparation adopts 5 μm C 18 The bonded phase preparation column uses methanol-water solution with a volume concentration of 75% as the elution system, and 6 pure compounds can be obtained after three-step separation. The specific process steps are as follows:
[0037] (1) Normal-phase silica gel column chromatography: extract the extract with 95% ethanol from Rhizoma chamaejasma, dissolve it in a mixture of chloroform and methanol, and add the same quality as the extract under constant stirring. 200-300 mesh column chromatography silica gel, mix the sample, and fully dry it at room temperature; then dry the sample, pass it through a 200-300 mesh silica gel column unde...
Embodiment 2
[0041] In this example, the 95% ethanol extract of Daphne chamaejasma was used as the raw material. The first step of normal phase column chromatography used 200-300 mesh silica gel, the second step of reverse-phase column chromatography used 40-50 μm ODS, and the third step HPLC preparation using 10 μm C 18 The bonded phase preparation column uses 50% acetonitrile-water solution as the elution system, and 6 kinds of pure compounds can be obtained after three-step separation. The specific process steps are as follows:
[0042] (1) Normal-phase silica gel column chromatography: extract the extract with 95% ethanol from Rhizoma chamaejasma, dissolve it in a mixture of chloroform and methanol, and add the same quality as the extract under constant stirring. 200-300 mesh column chromatography silica gel, mix the sample, and fully dry it at room temperature; then dry the sample, pass it through a 200-300 mesh silica gel column under normal pressure, and then use chloroform, chloro...
Embodiment 3
[0046] In this example, the 95% ethanol extract of Daphne chamaejasma was used as the raw material. The first step of normal phase column chromatography used 200-300 mesh silica gel, the second step of reverse-phase column chromatography used 40-50 μm ODS, and the third step HPLC preparation using 5 μm C 18 The bonded phase preparative column adopts 70% methanol-water solution as the eluting system, and 6 kinds of pure compounds can be obtained after three-step separation. The specific process steps are as follows:
[0047] (1) Normal-phase silica gel column chromatography: extract the extract with 95% ethanol from Rhizoma chamaejasma, dissolve it in a mixture of chloroform and methanol, and add the same quality as the extract under constant stirring. 200-300 mesh column chromatography silica gel, mix the sample, and fully dry it at room temperature; then dry the sample, pass it through a 200-300 mesh silica gel column under normal pressure, and then use chloroform, chlorofor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com