Glucokinase activator containing glucosamide and pyridine structure and application of glucokinase activator
A technology of compounds, acetyl groups, used in organic chemistry, metabolic diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
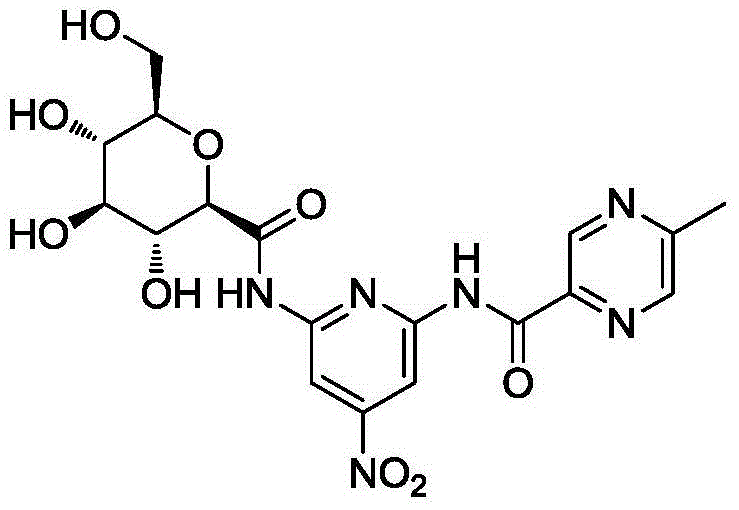
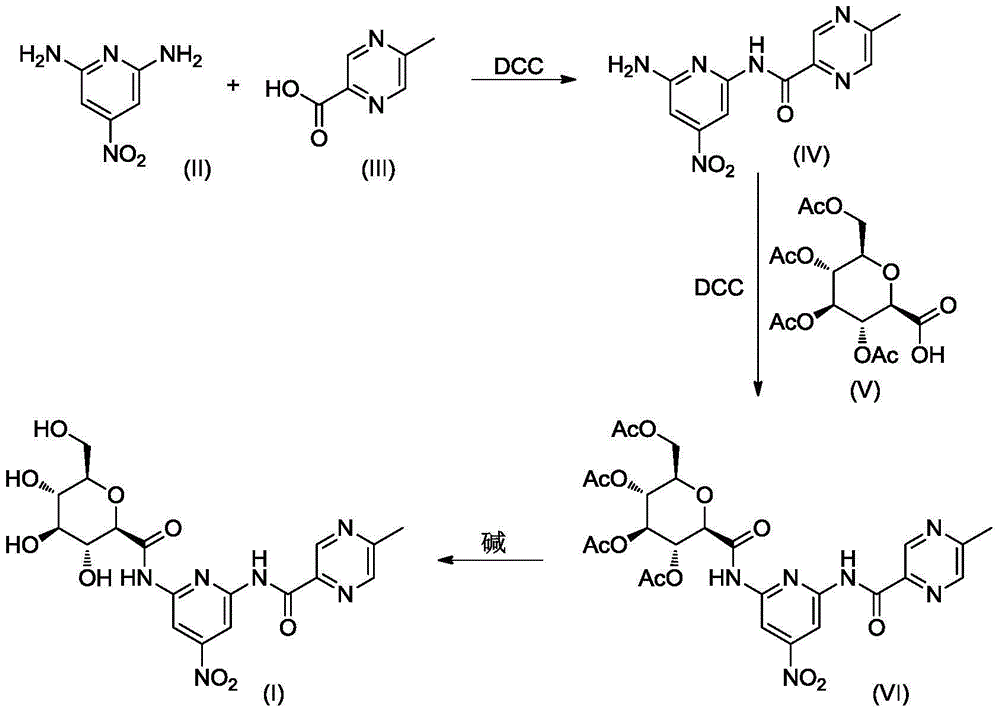
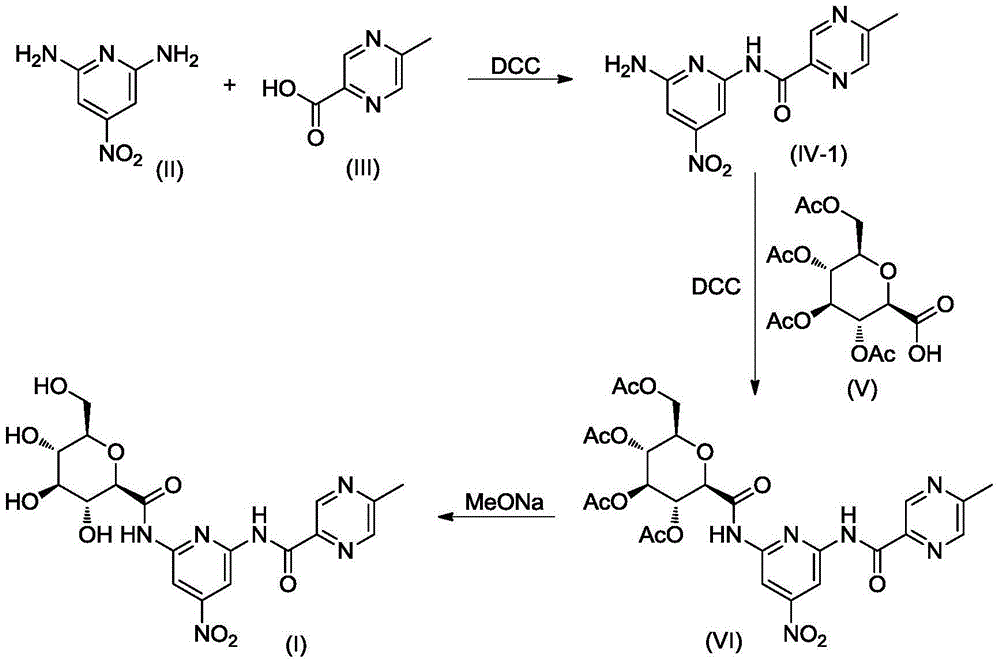
[0023] The synthesis of embodiment 1 compound I
[0024]
[0025] A. Synthesis of Compound IV
[0026] Compound II 1.54g (10mmol) and Compound III 1.38g (10mmol) were dissolved in 20mL of dry THF, stirred at room temperature, DCC 2.48g (12mmol) and 4-dimethylaminopyridine (DMAP) 0.50g were added, and the reaction mixture was then After stirring overnight at room temperature, the reaction was complete by TLC check. After cooling the mixture was poured into 100 mL ice water, using 50 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV, a white solid, ESI-MS, m / z=275 ([M+H] + ).
[0027] B. Synthesis of Compound VI
[0028] Compound IV 1.64g (6mmol) and compound V 2.26g (6mmol) were dissolved in 30mL dr...
Embodiment 2
[0031] The synthesis of embodiment 2 reference compound D-1
[0032]
[0033] A. Synthesis of Compound IV-2
[0034] Compound II-21.09g (10mmol) and compound III-21.24 (10mmol) were dissolved in 20mL dry THF, stirred at room temperature, DCC 2.48g (12mmol) and 4-dimethylaminopyridine (DMAP) 0.50g were added, and the reaction mixture After stirring overnight at room temperature, TLC showed the reaction was complete. After cooling the mixture was poured into 100 mL ice water, using 50 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV-2, a white solid, ESI-MS, m / z=216 ([M+H] + ).
[0035] B. Synthesis of Compound VI-2
[0036] Compound IV-21.29g (6mmol) and Compound V 2.26g (6mmol) were dissolved i...
Embodiment 3
[0039] Activation of the compound of embodiment 3 to glucokinase in vitro
[0040] In vitro glucokinase test
[0041] The in vitro activity of the glucokinase activators of the present invention was evaluated in two independent tests: with EC 50 Test to evaluate the potency of each compound at a fixed, physiologically relevant concentration of glucose, and the glucose S at a fixed, near-saturating (if possible) concentration of the compound 0.5 Test to evaluate its Vm and S for glucose 0.5 role. For each of these tests, glucokinase activity was estimated by monitoring the increase in absorbance at 340 nm in a coupled assay system containing NAD+ and glucose 6-phosphate dehydrogenase. Assays were performed at 30°C using a thermostatically controlled absorbance plate reader and clear, 96-well, flat bottom, polystyrene plates (Costar 3695, Coming). Each 50 μL test mixture contains 10 mM K+MOPS, pH 7.2, 2 mM MgCl 2 , 50mM KCl, 0.01% Triton X-100, 2% DMSO, 1mM DTT, 1mM ATP, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com