Synthesis method and application of platinum (II) complex using 2-benzoylpyridine as ligand
A technology of benzoylpyridine and its synthesis method, which is applied in the field of medicine, can solve the problems of clinical application limitation, low water solubility, DNA structure damage, etc., and achieve the effect of good selectivity, low toxicity, and significant in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Accurately weigh 0.5 mmol of 2-benzoylpyridine and 0.5 mmol of dichlorobis(dimethylsulfoxide) platinum (II), and dissolve 2-benzoylpyridine in 37 mL of 100% ( volume) in methanol, dichlorobis(dimethylsulfoxide) platinum (II) was dissolved in 9.5mL of water, the two solutions were mixed, reacted at 78°C for 24h, concentrated and evaporated to remove most of the solvent (solvent added 89% of the amount), cooled to room temperature, and stood still, and a yellow solid product was precipitated (yield 96%).
[0036] The resulting yellow solid product is identified:
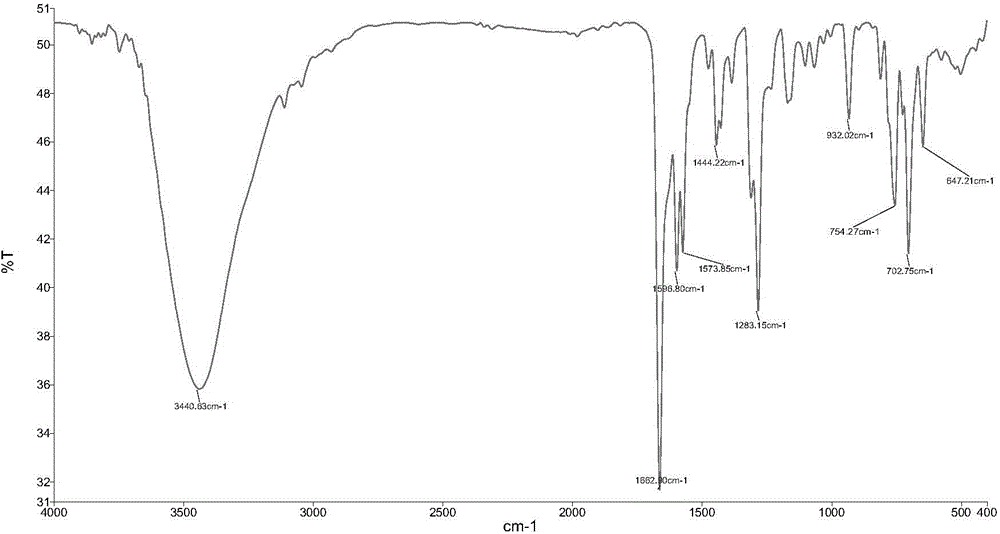
[0037] (1), infrared spectrum, its spectrogram is as follows figure 1 shown.
[0038] IR(KBr):3441,1663,1598,1574,1444,1283,932,754,703,647cm -1 .
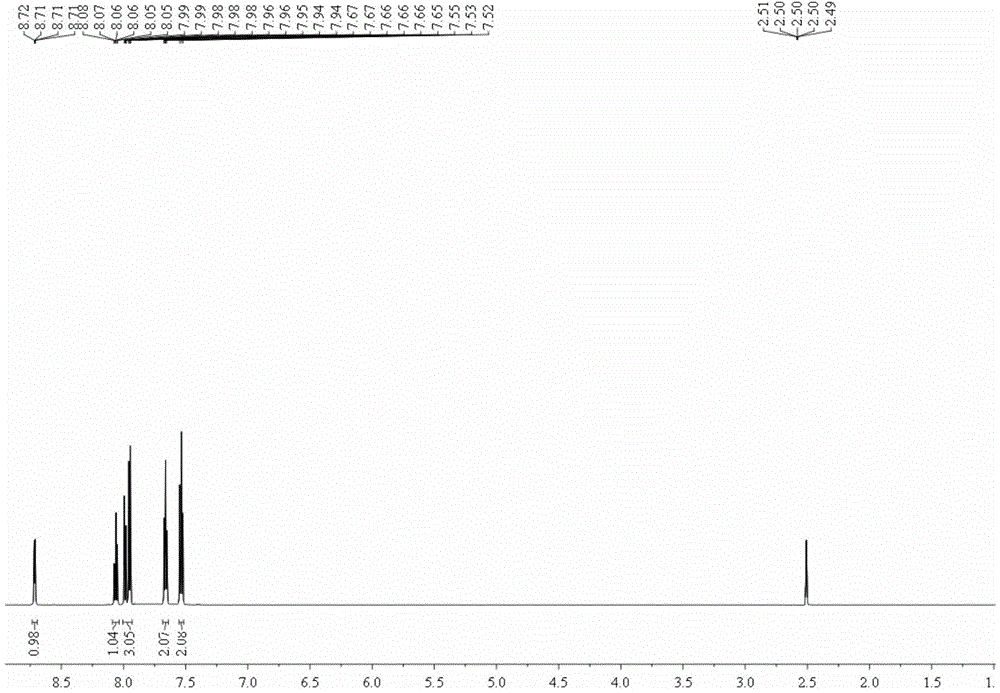
[0039] (2), proton nuclear magnetic resonance spectrogram, its spectrogram is as figure 2 shown.
[0040] 1 H NMR (600MHz, DMSO-d 6 )δ8.73–8.69(m,1H),8.06(td,J=7.7,1.7Hz,1H),8.00–7.93(m,3H),7.68–7.64(m,2H),7.53(t,J= 7.8Hz, 2H).
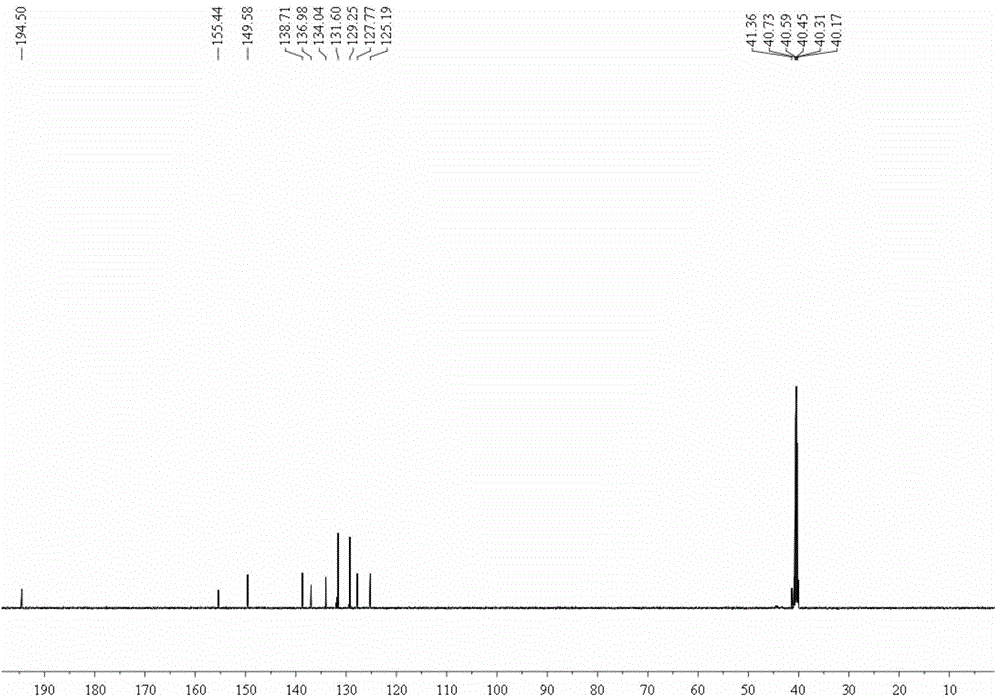
[0041] (3), carbon nuclear ...
Embodiment 2
[0052] Accurately weigh 0.5 mmol of 2-benzoylpyridine and 0.5 mmol of dichlorobis(dimethylsulfoxide) platinum (II), and dissolve 2-benzoylpyridine in 56 mL of 65% ( (volume) methanol and 75% (volume) ethanol (the volume ratio of 65% (volume) methanol and 75% (volume) ethanol is 5:8), dichloro two (dimethyl sulfoxide) Dissolve platinum (II) in a mixed solution of 25mL of water and 98% (volume) methanol (the volume ratio of water and 98% (volume) methanol is 1:98), mix the two solutions, react at 60°C for 32h, and concentrate After most of the solvent was removed by evaporation (85% of the solvent addition), it was cooled to room temperature and left to stand, and a yellow-green solid product (yield 84%) was separated out, and the obtained yellow solid product was determined to be the compound shown in the above formula (I) through structural characterization. compound.
Embodiment 3
[0054] Accurately weigh 0.5mmol of 2-benzoylpyridine and 0.5mmol of dichlorobis(dimethylsulfoxide)platinum(II), add the two to a 20cm Pyrex thick-walled glass tube with one end closed Add dropwise 80% (volume) methanol 3.5mL, dropwise add dimethyl sulfoxide 0.8mL, freeze the mixture with liquid nitrogen, seal its open end under vacuum conditions, mix well and place at 50°C Reacted in an oven for 72 hours to obtain a yellow solid product (yield 78%), which was determined to be the compound represented by the above formula (I) through structural characterization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com