Fluorene organic electroluminescent material and preparation method thereof
An electroluminescent material and luminescent technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems that luminescent materials cannot meet the requirements of OLED use, and achieve improved efficiency, increased yield, and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the synthesis of compound 001
[0034] The specific synthetic route is shown in the following formula:
[0035]
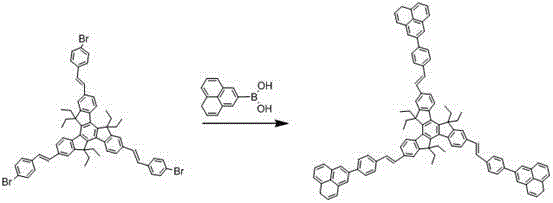
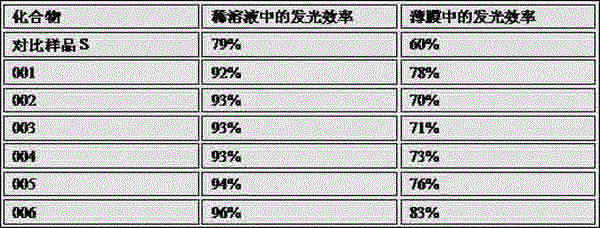
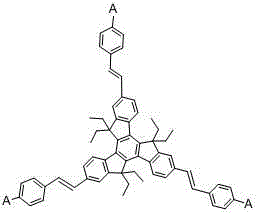
[0036] Add 10.54g (10mmol) of bromostyryl indenofluorene derivatives, 6.88g (40mmol) of 2-naphthalene boronic acid, 3.18g (30mmol) of sodium carbonate, 200ml of tetrahydrofuran and 100ml of water into a three-necked flask, degas, and add four three Phenylphosphopalladium 0.12g (0.10mmol), heated to 70°C, reacted for 24 hours, cooled to room temperature, filtered with suction, washed the filter cake with water, ethanol and ether, and dried to obtain star-shaped indeno 8.96 g of fluorene olefin derivatives, the yield is more than 75%, and the HPLC purity is greater than 98%. Mass spectrum: Calculated value is 1195.61; found value is 1195.62. Elemental analysis: Calculated value: C: 93.42%; H: 6.58%; Tested value: C: 93.41%; H: 6.59%.
[0037]
Embodiment 2
[0038] Embodiment 2: the synthesis of compound 002
[0039] The specific synthetic route is shown in the following formula:
[0040]
[0041]Add 10.54g (10mmol) of bromostyrylindenofluorene derivatives, 11.07g (45mmol) of 2-pyrenylboronic acid, 3.39g (32mmol) of sodium carbonate, 200ml of tetrahydrofuran and 100ml of water into a three-necked flask, degas, and add four Triphenylphosphopalladium 0.14g (0.12mmol), heated to 72°C, reacted for 25 hours, cooled to room temperature, filtered with suction, washed the filter cake with water, ethanol and ether, and dried to obtain star-shaped indene 10.77 g of fluorene olefin derivatives, the yield is over 76%, and the HPLC purity is greater than 98%. Mass spectrum: Calculated value 1417.85; found value 1417.83. Elemental analysis: Calculated value: C: 94.03%; H: 5.97%; Tested value: C: 94.04%; H: 5.96%.
[0042]
Embodiment 3
[0043] Embodiment 3: the synthesis of compound 003
[0044] The specific synthetic route is shown in the following formula:
[0045]
[0046] Add 10.54g (10mmol) of bromostyryl indenofluorene derivatives, 14.35g (50mmol) of N-phenylcarbazolylboronic acid, 3.60g (34mmol) of sodium carbonate, 200ml of tetrahydrofuran and 100ml of water into a three-necked flask and degas , add 0.16g (0.14mmol) tetrakistriphenylphosphopalladium, heat up to 74°C, react for 26 hours, cool to room temperature, after the solid precipitates, filter with suction, wash the filter cake with water, ethanol and ether, and dry to obtain 11.56 g of star-shaped indenofluorene olefin derivatives, the yield is over 75%, and the HPLC purity is over 98%. Mass Spectrum: Calculated value: 1541.01; Tested value: 1541.03. Elemental analysis: Calculated value: C: 91.19%; H: 6.08%; N: 2.73%; Tested value: C: 91.18%; H: 6.09%; N: 2.73%.
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com