A kind of preparation method of amino-terminated polymer
An amino-terminated polymer and free radical technology, which can be used in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc. High reactivity, convenient synthesis and purification, and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1, the preparation of ATRP initiator is as follows:
[0023] (1) Dissolve 1g of p-nitroaniline and 2g of triethylamine in 50mL of dichloromethane and place in a 150mL single-necked round bottom flask at room temperature for 1h;
[0024] (2) 2g of 2-bromoisobutyryl bromide was dissolved in 5mL of dichloromethane, added dropwise into the reaction vessel in step (1), and stirred at room temperature for 24h;
[0025] (3) until the reaction is finished, the reaction solution is suction filtered to remove triethylamine hydrochloride, and the filtrate passes through a silica gel column to obtain the initiator, and its structural formula is as follows:
[0026]
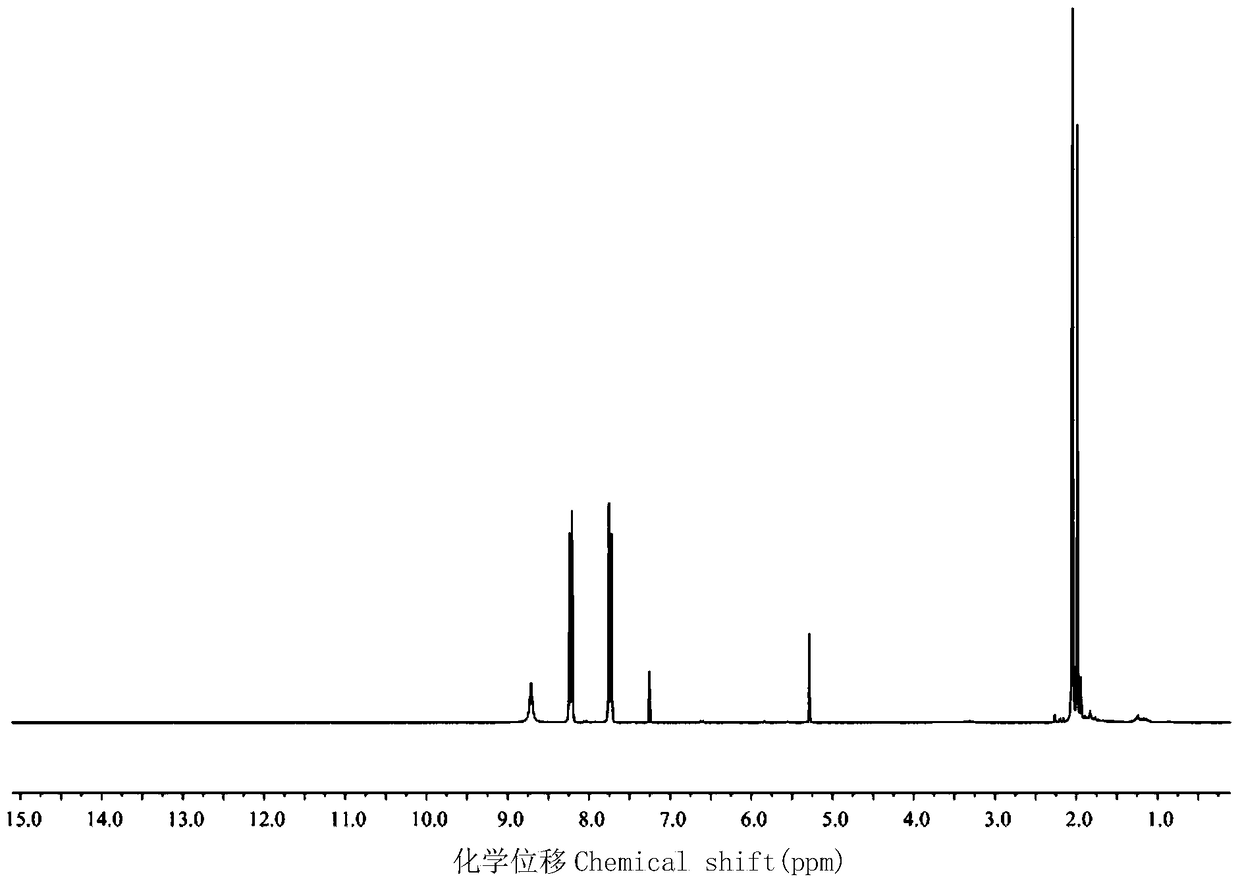
[0027] Its NMR spectrum is as figure 1 shown.
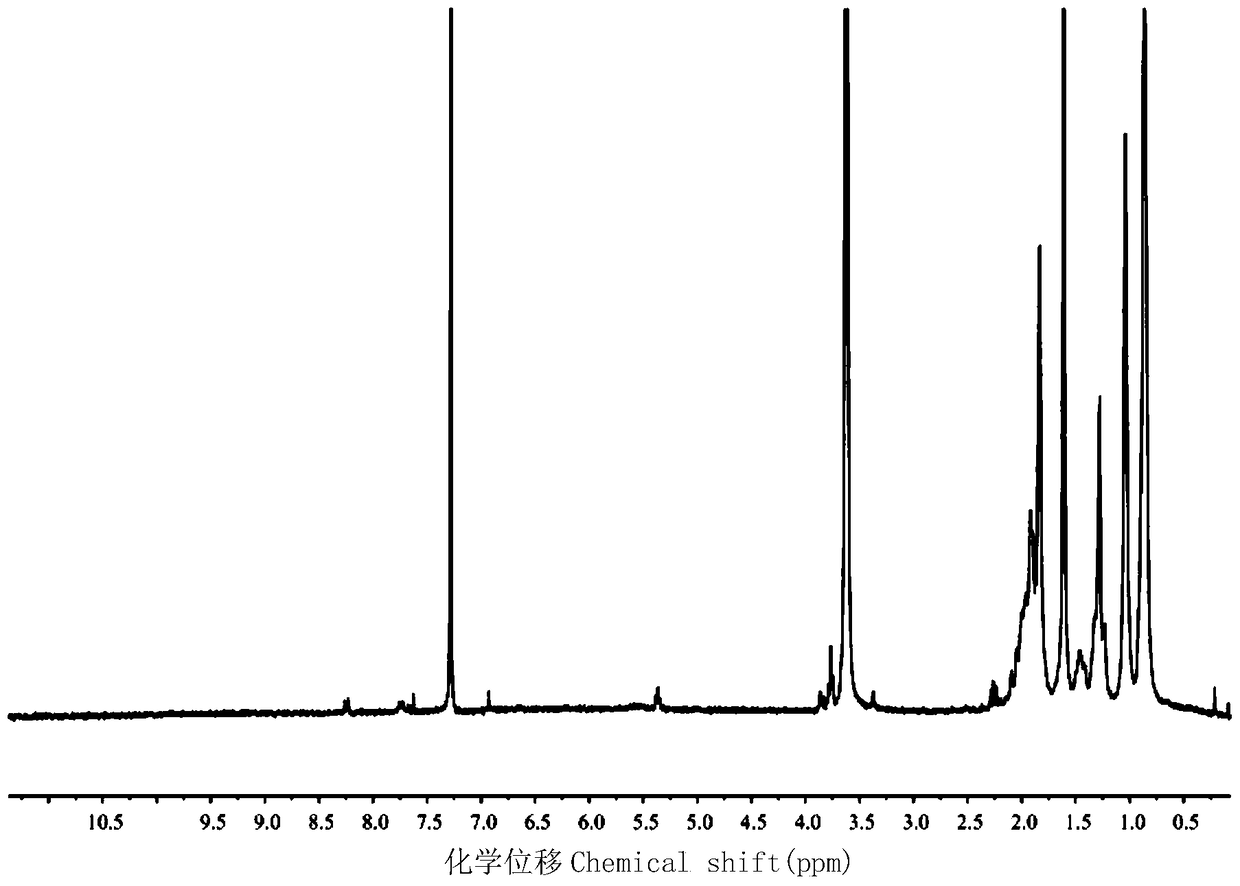
[0028] 2. The preparation of the amino-terminated polymer (taking polymethyl methacrylate as an example) is as follows:
[0029] (1) In a schlenk tube, the initiator (0.053g, 1.850×10 -4 mol), methyl methacrylate (0.463g, 4.625×10 -3 mol), 2-2 bipyridine (0.087g, ...
Embodiment 2
[0032] 1, the preparation of ATRP initiator is as follows:
[0033] (1) Dissolve 1.5g of p-nitroaniline and 2.5g of triethylamine in 50mL of dichloromethane and place in a 150mL single-necked round bottom flask at room temperature for 1.5h;
[0034] (2) 3g of 2-bromoisobutyryl bromide was dissolved in 10mL of dichloromethane, added dropwise into the reaction vessel in step (1), and stirred at room temperature for 24h;
[0035] (3) After the reaction is completed, the reaction solution is suction-filtered to remove triethylamine hydrochloride, and the filtrate is passed through a silica gel column to obtain the target product.
[0036] 2. The preparation of the amino-terminated polymer (taking polymethyl methacrylate as an example) is as follows:
[0037] (1) In a schlenk tube, the initiator (0.053g, 1.850×10 -4 mol), methyl methacrylate (3.704g, 3.7×10 -2 mol), 2-2 bipyridine (0.029g, 1.83×10 -4 mol) was dissolved in tetrahydrofuran, and the oxygen was removed through 3 cy...
Embodiment 3
[0040] 1, the preparation of ATRP initiator is as follows:
[0041] (1) Dissolve 2g of p-nitroaniline and 3g of triethylamine in 50mL of dichloromethane and place in a 150mL single-necked round bottom flask and stir at room temperature for 2h;
[0042] (2) 3g of 2-bromoisobutyryl bromide was dissolved in 10mL of dichloromethane, added dropwise into the reaction vessel in step (1), and stirred at room temperature for 24h;
[0043] (3) After the reaction is completed, the reaction solution is suction-filtered to remove triethylamine hydrochloride, and the filtrate is passed through a silica gel column to obtain the target product.
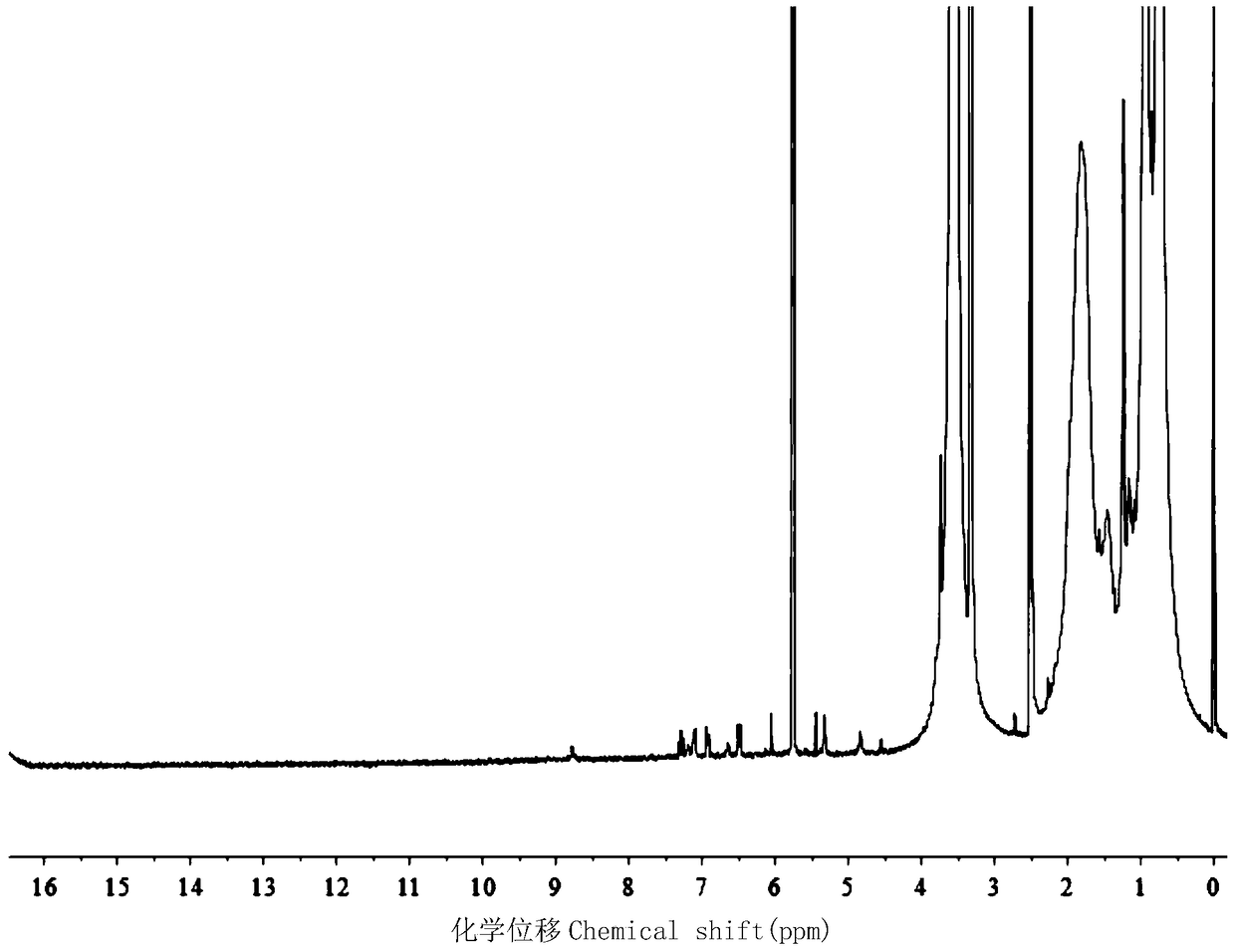
[0044] 2. The preparation of the amino-terminated polymer (taking polyhydroxyethyl methacrylate as an example) is as follows:
[0045] (1) In a schlenk tube, the initiator (0.053g, 1.850×10 -4 mol), hydroxyethyl methacrylate (1.203g, 9.25×10 -3 mol), 2-2 bipyridine (0.087g, 5.49×10 -4 mol) was dissolved in methanol and subjected to three cycles o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com