D (A1-pi-A2) 2 type conjugated small molecule based on diaryl and indacene fused ring unit and preparation method of D (A1-pi-A2) 2 type conjugated small molecule
A small molecule, condensed ring technology, used in semiconductor/solid-state device manufacturing, electrical components, organic chemistry, etc., to achieve excellent near-infrared absorption performance, high short-circuit current, and improved sunlight utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Conjugated Small Molecule IDT (DPP-TDRCN) 2 Synthesis
[0044] The synthetic route and synthetic steps of this conjugated small molecule are as follows:
[0045]
[0046] 1. Synthesis of compound 2
[0047] In a 250mL there-necked flask, compound 1 (1.00g, 1.06mmol), 5-formyl-2-thiopheneboronic acid (0.51g, 2.12mmol), tetrakis (triphenylphosphine) palladium (25mg, 0.02mmol) were added successively, Sodium bicarbonate (0.53 g, 6.36 mmol) solution (15 mL distilled water) and 120 mL tetrahydrofuran. Under nitrogen protection, the reaction was refluxed at 85 °C for 24 h, the reaction was stopped, and the reaction was cooled to room temperature. The reaction solution was transferred to a separatory funnel, 50 mL of distilled water was added, extracted with dichloromethane, dried over anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to remove the organic solvent, and the obtained crude product was represented by petroleum ether (PE): dichlorom...
Embodiment 2
[0055] Conjugated Small Molecule IDT (TID-TDRCN) 2 Synthesis
[0056] The synthetic route and synthetic steps of this conjugated small molecule are as follows:
[0057]
[0058] 1. Synthesis of compound 8
[0059] In a 250mL three-necked flask, compound 7 (10g, 61.40mmol), 2-ethylhexylamine (11.9g, 92.10mmol), copper powder (0.20g, 3.08mmol), iodide (0.59g, 3.08g) were added successively mmol), potassium phosphate (26.06 g, 122.80 mmol), N,N-dimethylethanolamine (120 mL), under nitrogen protection, the reaction was stirred at 80° C. for 48 h. The reaction was stopped, cooled, and filtered with suction. The obtained filtrate was subjected to rotary distillation under reduced pressure to remove the solvent, and the residue was distilled under reduced pressure to obtain compound 8 (5.2 g, 40%) as a brownish-yellow liquid. 1 H NMR (400MHz, CDCl 3 )δ: 7.18(dd, 1H), 6.65(dd, 1H), 5.95(s, 1H), 3.61(s, 1H), 3.01(d, 2H), 1.63(m, 1H), 1.30-1.35(m ,8H),0.92(m,6H).
[0060] 2. Sy...
experiment example 3
[0075] Conjugated small molecule IDT (DPP-TDRCN) prepared in Example 1 2 and the IDT (TID-TDRCN) prepared in Example 2 2 Performance characterization:
[0076] of the synthesized compounds 1 H NMR spectrum was determined by Bruker Dex-300 NMR or 400NMR instrument; mass spectrum (MS) was obtained by analyzing and testing the MALDI-TOF mass spectrometer of Bruker Bifiex; thermogravimetric (TGA) curve was obtained by thermal analyzer (model Perking-El TGA), tested at a heating rate of 20 °C / min; differential scanning calorimetry (DSC) curve was tested by TA DSCQ10 analyzer, under nitrogen atmosphere, tested at a heating rate of 20 °C / min; UV-Vis absorption spectrum by Shimadzu UV-800 UV-Vis spectrophotometer was used to measure; the cyclic voltammetry curve was measured by CHI630E electrochemical analyzer.
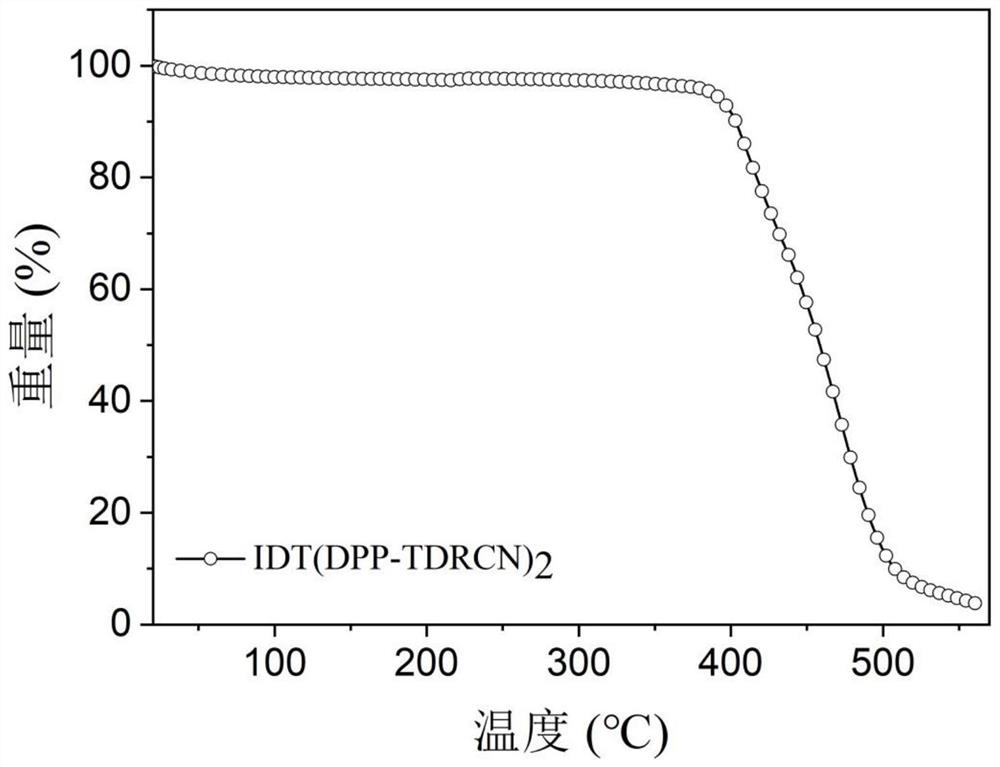
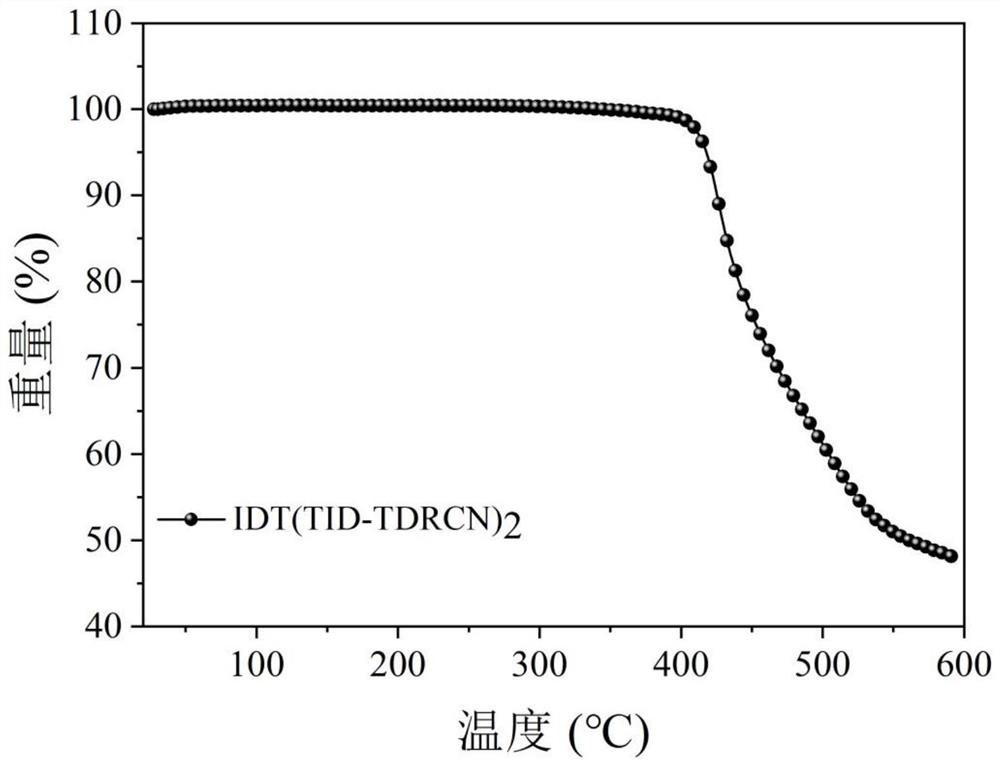
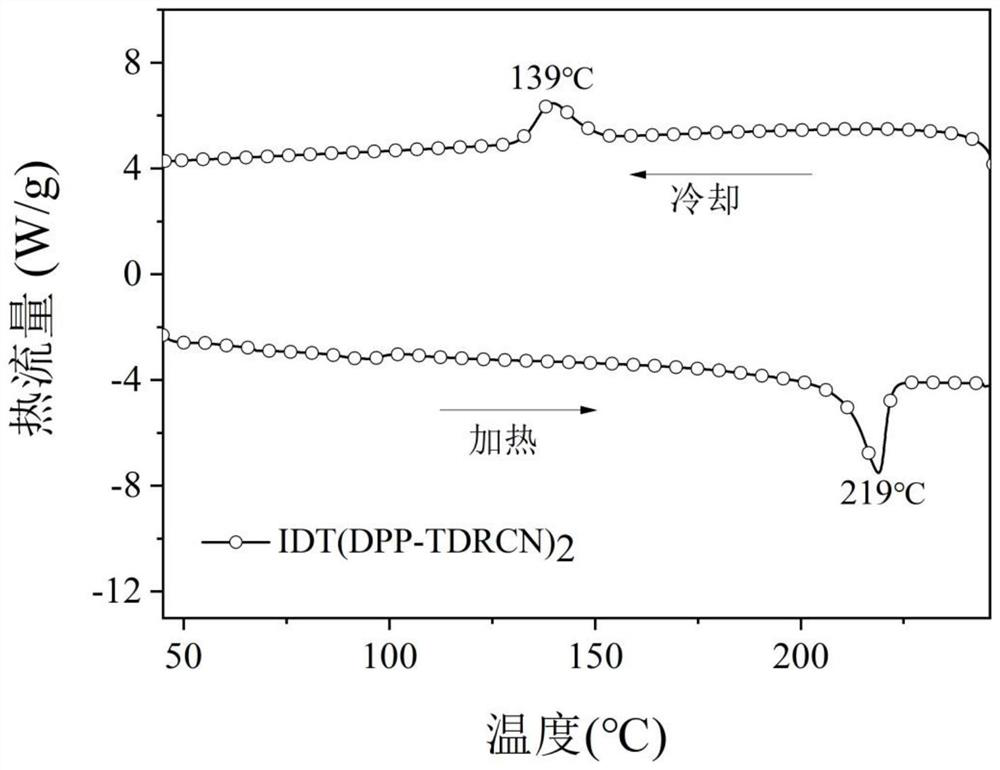
[0077] 1. Conjugated small molecule IDT (DPP-TDRCN) 2 and IDT (TID-TDRCN) 2 Thermal Stability Determination of
[0078] figure 1 and figure 2 Shown are the conjugate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com