An aromatic amine derivative containing bifluorene and its organic electroluminescent device
A technology of electroluminescent devices and derivatives, applied in the fields of electro-solid devices, organic chemistry, light-emitting materials, etc., can solve the problems of poor stability, low hole mobility, short life of organic electroluminescent devices, etc., and achieve good stability. properties, high hole mobility, and improved luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] Compound preparation
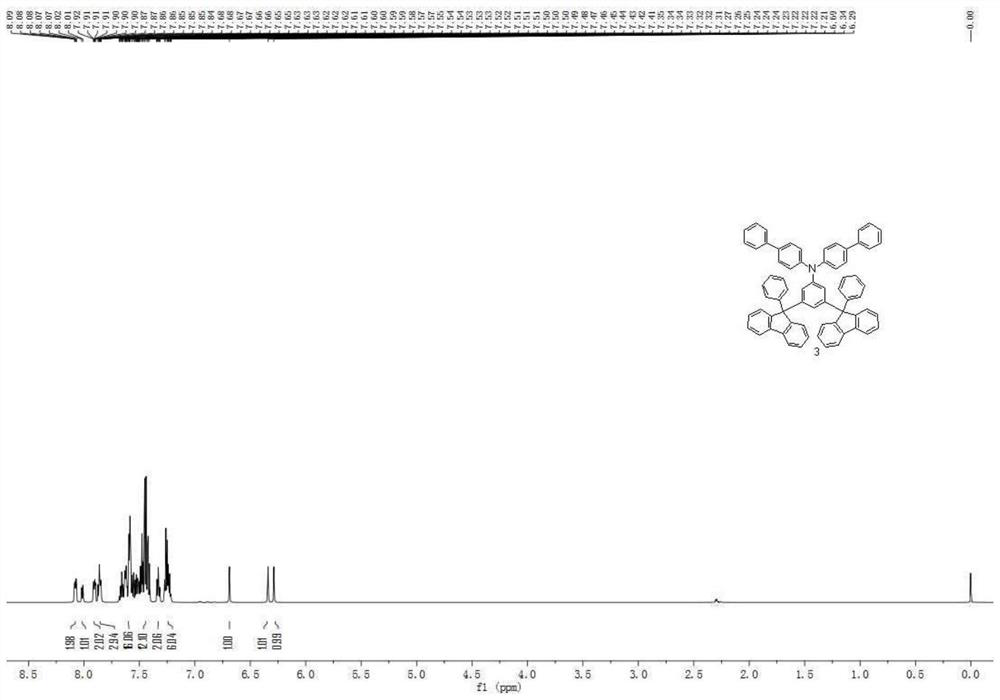
[0098] Synthesis Example 1: Preparation of Compound 3
[0099]
[0100] (1) Add p-chlorobenzoic acid (12.5g, 80mmol), benzoylformic acid (36.0g, 240mmol), silver carbonate (66.18g, 240mmol), palladium trifluoroacetate (2.66g, 8mmol) into a 1L reaction flask , anhydrous dipotassium hydrogen phosphate (27.9g, 160mmol), ethylene glycol dimethyl ether (480ml), reacted under nitrogen protection at 150°C for 24 hours, cooled to room temperature after the reaction, extracted with ethyl acetate, combined organic phase, the organic phase was successively washed with water, dried over anhydrous magnesium sulfate, concentrated, and subjected to column chromatography (silica gel, dichloromethane) to obtain compound a-1. Mass 17.4g, yield 68%.
[0101] (2) After adding 2-chlorobiphenyl (14.1g, 75mmol) and tetrahydrofuran (250ml) into a 1L reaction flask, the reaction system was cooled to -78°C, and then a n-hexane solution of n-butyllithium (2.5 M, 30ml)...
Embodiment 1
[0128] Embodiment 1: Preparation of organic electroluminescent device 1
[0129] ITO is used as the anode on the glass substrate; 1T-NATA is vacuum evaporated on the anode as a hole injection layer, and the evaporation thickness is 60nm; the compound 3 of the present invention is vacuum evaporated on the hole injection layer as a hole transport layer, Evaporation thickness is 30nm; vacuum evaporation AND:BD-1(98:2) is used as the light-emitting layer on the hole transport layer, and the evaporation thickness is 20nm; vacuum evaporation Alq on the light-emitting layer 3 As the electron transport layer, the evaporation thickness is 30nm; on the electron transport layer, LiF is vacuum evaporated as the electron injection layer, and the evaporation thickness is 1nm; on the electron injection layer, Al is vacuum evaporated as the cathode, and the evaporation thickness is 150nm.
[0130] Device structure of organic electroluminescent device 1: ITO / 1T-NATA (60nm) / compound 3 (30nm) / AN...
Embodiment 2~20
[0131] Embodiments 2-20: Preparation of Organic Electroluminescent Devices 2-20
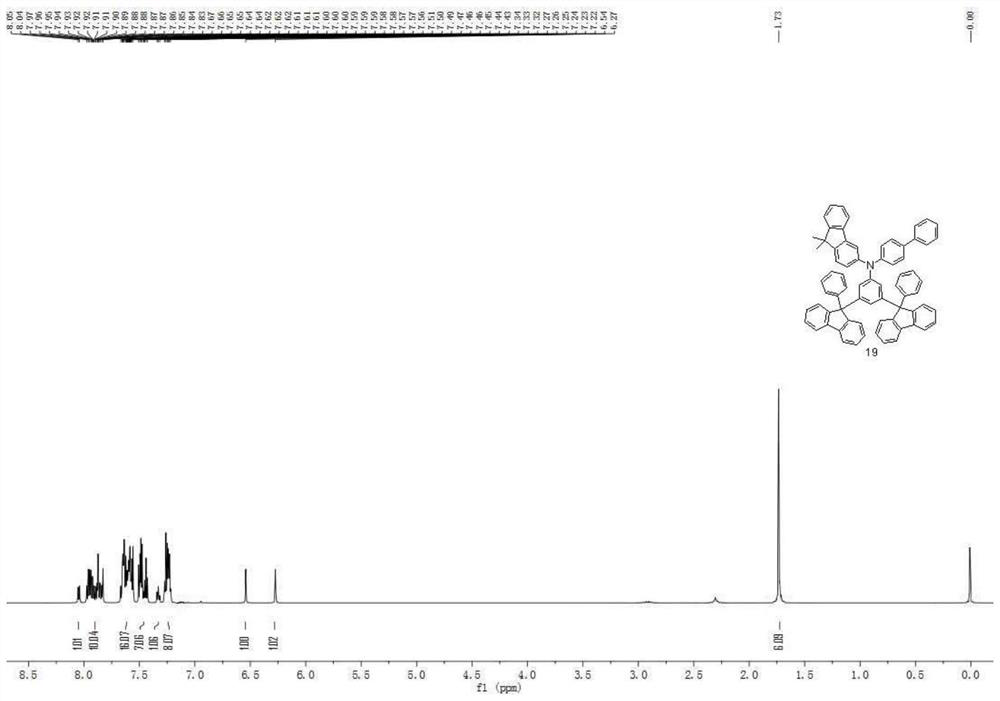
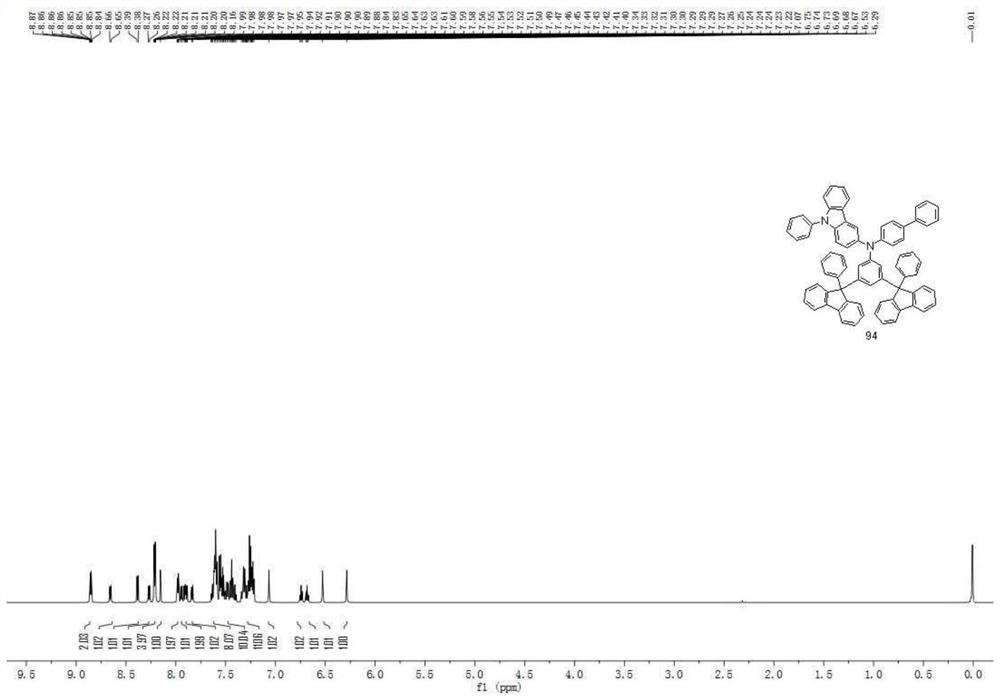
[0132] Compound 3 in the hole transport layer of Example 1 was replaced by Compound 4, Compound 9, Compound 19, Compound 25, Compound 30, Compound 39, Compound 74, Compound 80, Compound 94, Compound 100, Compound 104, Compound 113 , compound 130, compound 134, compound 136, compound 158, compound 166, compound 169, compound 171, and other steps were the same to obtain organic electroluminescent devices 2-20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com