A kind of mncoge-based ferromagnetic martensitic phase change material and its preparation method and application
A technology of martensitic phase transformation and ferromagnetism, applied in the direction of magnetic materials, magnetic objects, electrical components, etc., to achieve the effects of easy control of composition, no rare earth elements, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
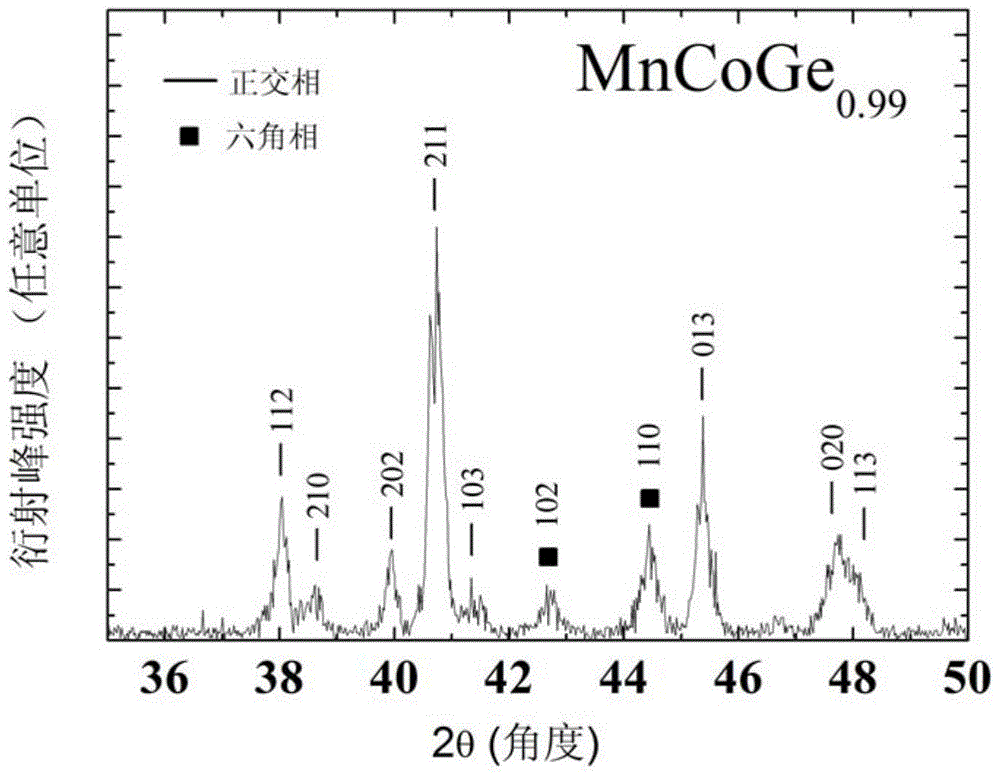
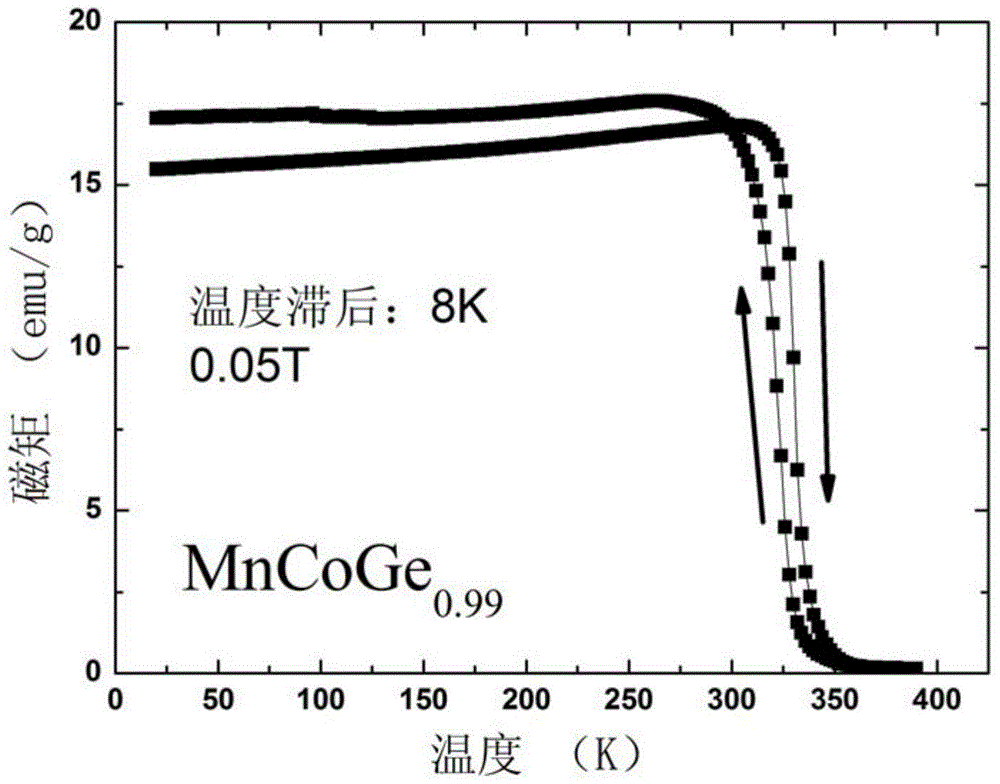
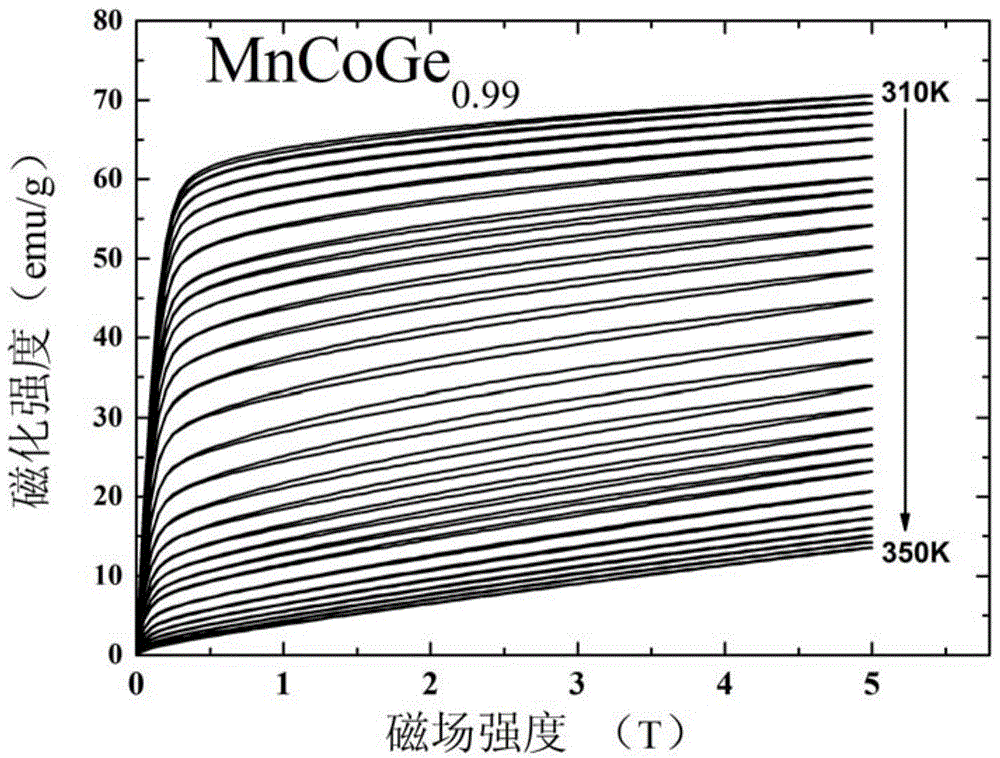
[0038] This embodiment according to the chemical formula MnCoGe 0.99 To prepare the phase change material of the present invention, the specific preparation method is as follows:
[0039] (1) According to the chemical formula MnCoGe 0.99 Prepare the raw materials, put the prepared raw materials into the electric arc furnace, and evacuate to 3×10 -3 Above Pa, after cleaning once with high-purity argon (purity 99.996wt%), under the protection of 1 atmospheric pressure of high-purity argon (purity 99.996wt%), the arc is started, and the melting temperature is 2000 ℃. After smelting, cool to room temperature in a copper crucible to obtain a cast alloy ingot.
[0040] (2) Wrap the alloy ingots prepared in step (1) with metal molybdenum sheets, and seal them in a vacuum quartz tube (the vacuum degree is 1×10 -4 Pa), after annealing at 875°C for 6 days, the quartz tube was taken out, cooled to room temperature naturally, and the quartz tube was broken to obtain MnCoGe 0.99 Phase...
Embodiment 2
[0048] This embodiment according to the chemical formula MnCoGe 0.97 To prepare the phase change material of the present invention, the specific preparation method is as follows:
[0049] (1) According to the chemical formula MnCoGe 0.97 Prepare the raw materials, put the prepared raw materials into the electric arc furnace, and evacuate to 3×10 -3 Above Pa, after cleaning twice with high-purity argon (purity 99.996wt%), under the protection of 1 atmospheric pressure of high-purity argon (purity 99.996wt%), the arc is started, and the melting temperature is 2000 ℃. After smelting, cool to room temperature in a copper crucible to obtain a cast alloy ingot.
[0050] (2) Wrap the alloy ingots prepared in step (1) with metal molybdenum sheets, and seal them in a vacuum quartz tube (the vacuum degree is 1×10 -4 Pa), after annealing at 875°C for 6 days, the quartz tube was taken out, cooled to room temperature naturally, and the quartz tube was broken to obtain MnCoGe 0.97 Phas...
Embodiment 3
[0058] This embodiment according to the chemical formula MnCoGe 0.96 To prepare the phase change material of the present invention, the specific preparation method is as follows:
[0059] (1) According to the chemical formula MnCoGe 0.96 Prepare the raw materials, put the prepared raw materials into the electric arc furnace, and evacuate to 3×10 -3 Above Pa, after cleaning twice with high-purity argon (purity 99.996wt%), under the protection of 1 atmospheric pressure of high-purity argon (purity 99.996wt%), the arc is started, and the melting temperature is 2000 ℃. After smelting, cool to room temperature in a copper crucible to obtain a cast alloy ingot.
[0060] (2) Wrap the alloy ingots prepared in step (1) with metal molybdenum sheets, and seal them in a vacuum quartz tube (the vacuum degree is 1×10 -4 Pa), after annealing at 875°C for 6 days, the quartz tube was taken out, cooled to room temperature naturally, and the quartz tube was broken to obtain MnCoGe 0.96 Phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com