Method for modifying surface of anode material Li1.2Ni0.13Co0.13Mn0.54O2 for lithium ion battery
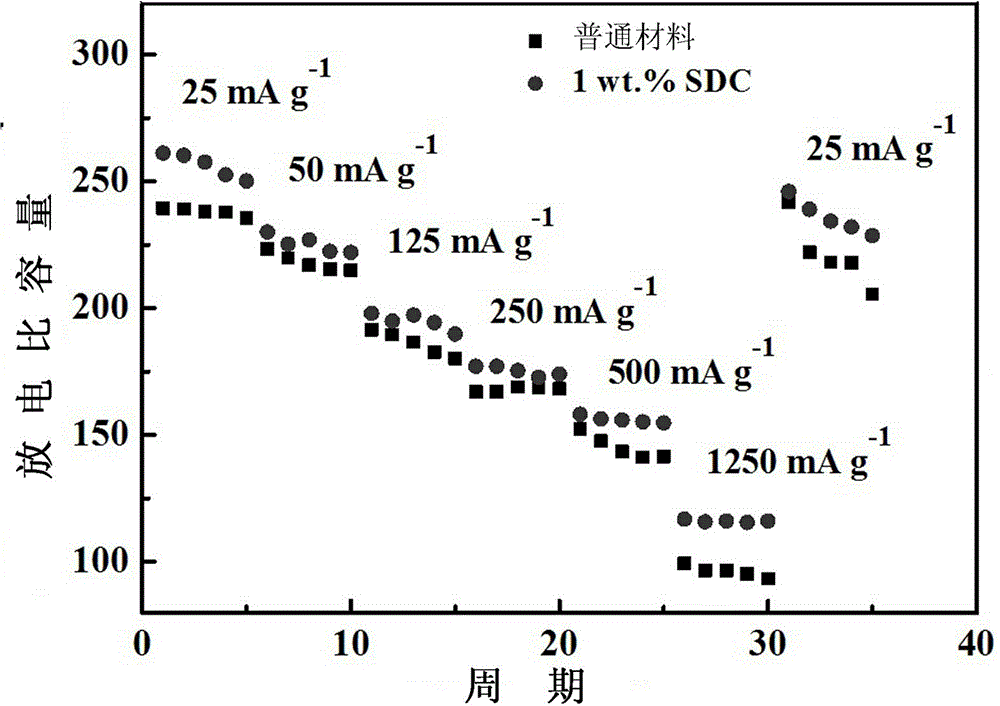
A technology for lithium-ion batteries and lithium-rich cathode materials, which is applied in battery electrodes, circuits, electrical components, etc., can solve the problems of large irreversible capacity loss during first charge and discharge, unfavorable lithium-ion battery cycle life, cycle performance and rate performance degradation, etc. , to alleviate disappearance and harmful side reactions, reduce irreversible capacity loss, and improve rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
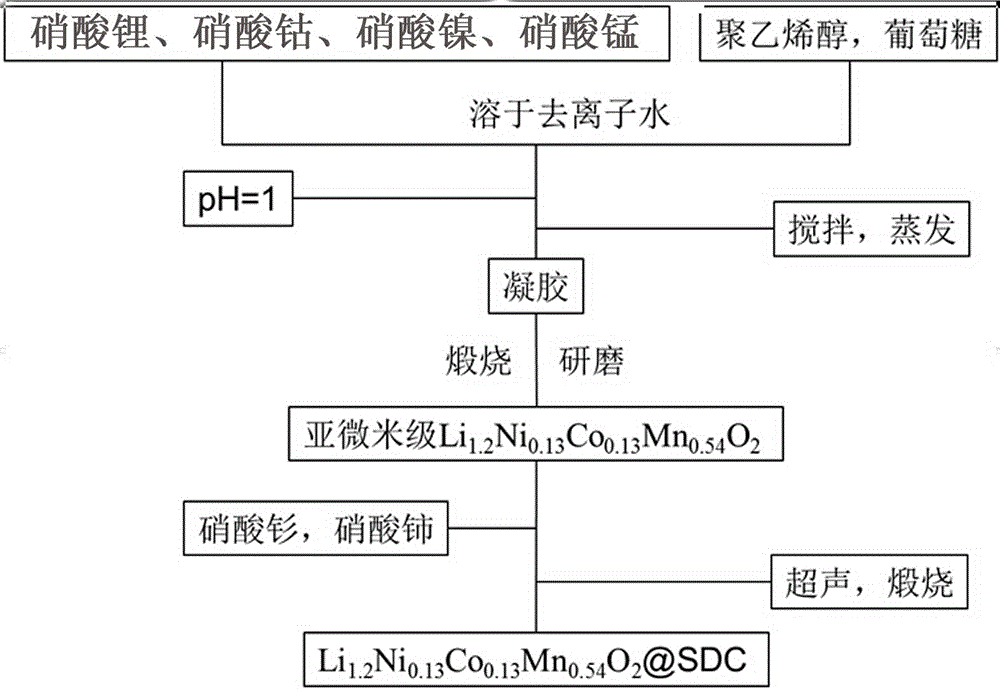
[0031] See figure 1 , a cathode material for Li-ion batteries Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 The surface modification method, comprises the following steps:
[0032] A. Submicron lithium-rich cathode material Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 Preparation of:
[0033] A1. Dissolve a total of 6g of lithium nitrate, cobalt nitrate, nickel nitrate and manganese nitrate in 50mL of deionized water according to the atomic molar ratio Li:Ni:Co:Mn=1.2:0.13:0.13:0.54, and then mix polyvinyl alcohol and A total of 0.8g of glucose was added to the obtained solution, wherein the mass ratio of polyvinyl alcohol and glucose mixture was 1:3, and the pH of the solution was adjusted with nitric acid to make the pH = 0.5, and the solution was stirred and evaporated to form a gel;
[0034] A2. Dry the above-mentioned gel in a drying oven at 110°C for 24 hours, and transfer the completely dried gel to a muffle furnace. The temperature of the muffle furnace is raised to 800°C at a r...
Embodiment 2
[0039] See figure 1 , a cathode material for Li-ion batteries Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 The surface modification method, comprises the following steps:
[0040] A. Submicron lithium-rich cathode material Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 Preparation of:
[0041] A1. Dissolve a total of 7.4g of lithium nitrate, cobalt nitrate, nickel nitrate and manganese nitrate in 60mL of deionized water according to the atomic molar ratio Li:Ni:Co:Mn=1.2:0.13:0.13:0.54, and then dissolve polyethylene A total of 1g of alcohol and glucose was added to the obtained solution, wherein: the mass ratio of polyvinyl alcohol and glucose mixture was 1:1, the pH of the solution was adjusted with nitric acid to make the pH of the solution = 1, and the solution was stirred and evaporated to form a gel;
[0042] A2. Dry the above-mentioned gel in a drying oven at 120°C for 18 hours, transfer the completely dried gel to a muffle furnace, and the muffle furnace heats up to 900°C at a ra...
Embodiment 3
[0048] See figure 1 , a cathode material for Li-ion batteries Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 The surface modification method, comprises the following steps:
[0049] A. Submicron lithium-rich cathode material Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 Preparation of:
[0050] A1. Dissolve 8.8g of lithium nitrate, cobalt nitrate, nickel nitrate and manganese nitrate in 70mL deionized water according to the atomic molar ratio Li:Ni:Co:Mn=1.2:0.13:0.13:0.54 to form a solution, and then polyethylene A total of 1.2 g of alcohol and glucose was added to the resulting solution, wherein the mass ratio of polyvinyl alcohol and glucose mixture was 3:1, the pH of the solution was adjusted with nitric acid to make the pH of the solution = 1.5, and the solution was stirred and evaporated to form a gel;
[0051] A2. Dry the above-mentioned gel in a drying oven at 130°C for 12 hours, transfer the completely dried gel to a muffle furnace, and the muffle furnace heats up to 1000°C at a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com