Preparation method of Zhongganling capsules and quality detection method of Zhongganling capsules
A technology of Chongganling and capsules, which is applied in the field of new preparation technology and quality inspection of the Chinese and Western medicine compound preparation "Chongganling Capsule", which can solve the problems of difficult control of preparation quality, destruction of active ingredients, long heating time, etc., and eliminate Bad effects, energy saving, process convenience effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
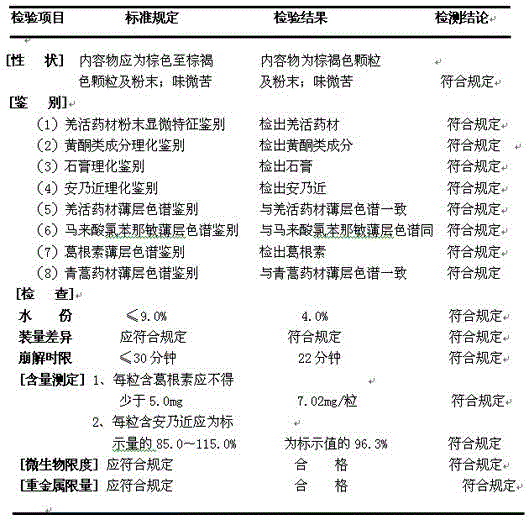
[0020] Extraction: take 4.69Kg of Chinese herbal medicines Ilex pubescens, 4.69Kg of Pueraria lobata, 3.12Kg of verbena, 2.5Kg of Radix isatidis, and 1.25Kg of Artemisia annua, crushed through 60 mesh, soaked in 975Kg of water for 1.5 hours, and then extracted with ultrasonic waves at a bath temperature of 65°C The filter was extracted at 40kHz for 45min, and then filtered to obtain a filtrate. Add 812.5Kg water to the filter residue, extract with an ultrasonic extractor at 35kHz for 40min at a bath temperature of 60°C, filter to obtain the second filtrate; combine the two extracts, and depressurize at a vacuum degree of 0.85-0.95Mpa and 60-70°C Concentrate to a relative density of 1.20 (60 ° C) extract to obtain water extract extract.
[0021] Drying: Spray-dry the water extract extract prepared above with a centrifugal spray dryer: the feed temperature of the extract is kept at 60°C-75°C; ℃~75℃; the rotating speed of the centrifugal atomizer is 40Hz, and the feeding speed i...
Embodiment 2
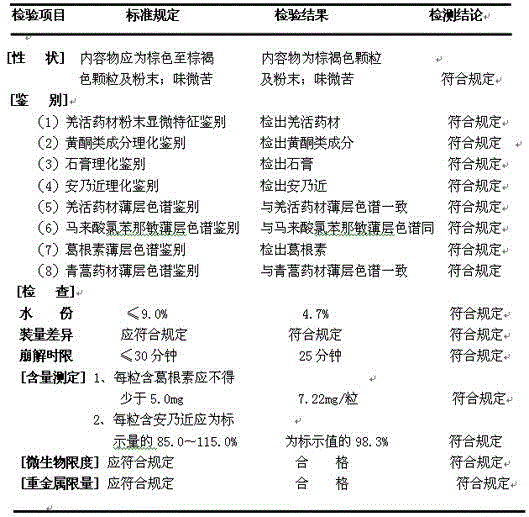
[0029] Extraction: Take 9.38Kg of Chinese herbal medicines Ilex pubescens, 9.38Kg of Pueraria lobata, 6.24Kg of verbena, 5.0Kg of Radix isatidis, and 2.50Kg of Artemisia annua, crush them through 60 meshes, soak them in 1950Kg of water for 2.0 hours, and then use ultrasonic waves at a bath temperature of 70°C. The extractor extracts at 40kHz for 50min, and then filters to obtain a filtrate. Add 1625Kg water to the filter residue, extract with an ultrasonic extractor at 40kHz for 40min at a bath temperature of 70°C, filter to obtain the second filtrate; combine the two extracts, and concentrate under reduced pressure at a vacuum degree of 0.85-0.95Mpa and 60-70°C To the relative density of 1.20 (60 ℃) extract, water extract extract.
[0030]Drying: Spray-dry the water extract extract prepared above with a centrifugal spray dryer: the feed temperature of the extract is kept at 60°C-75°C; ℃~75℃; the rotating speed of the centrifugal atomizer is 40Hz, and the feeding speed is 10m...
Embodiment 3
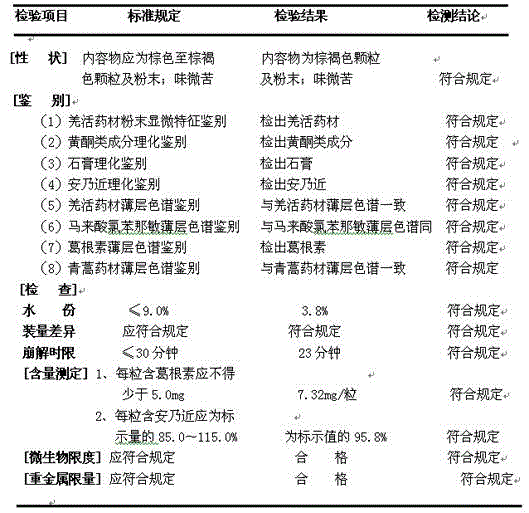
[0038] Extraction: Take 2.48Kg of Chinese herbal medicines Ilex pubescens, 2.48Kg of Pueraria lobata, 1.56Kg of verbena, 1.25Kg of Radix isatidis, and 0.63Kg of Artemisia annua, crush them through 60 meshes, soak them in 487Kg of water for 1.5 hours, and then use ultrasonic waves at a bath temperature of 70°C. The extractor extracts at 35kHz for 50min, and then filters to obtain a filtrate. Add 406Kg of water to the filter residue, extract with an ultrasonic extractor at 35kHz for 40min at a bath temperature of 60°C, and filter to obtain a secondary filtrate; combine the two extracts and concentrate under reduced pressure at a vacuum degree of 0.85-0.95Mpa at 60-70°C To the relative density of 1.20 (60 ℃) extract, water extract extract.
[0039] Drying: Spray-dry the water extract extract prepared above with a centrifugal spray dryer: the feed temperature of the extract is kept at 60°C-75°C; the spray tower is maintained at a temperature of 130°C-140°C, The temperature is 70°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com