Vaccine adjuvant and application thereof in preparation of Newcastle disease inactivating vaccine
An inactivated vaccine and Newcastle disease technology, which is applied in the field of preparation of Newcastle disease inactivated vaccine, can solve the problems of unsatisfactory immune effect of the Newcastle disease inactivated vaccine, and achieve the effect of good protection, good safety and prolonging the protection period.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
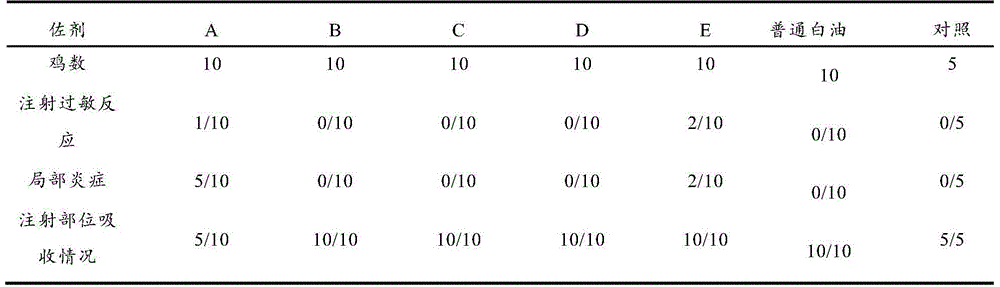
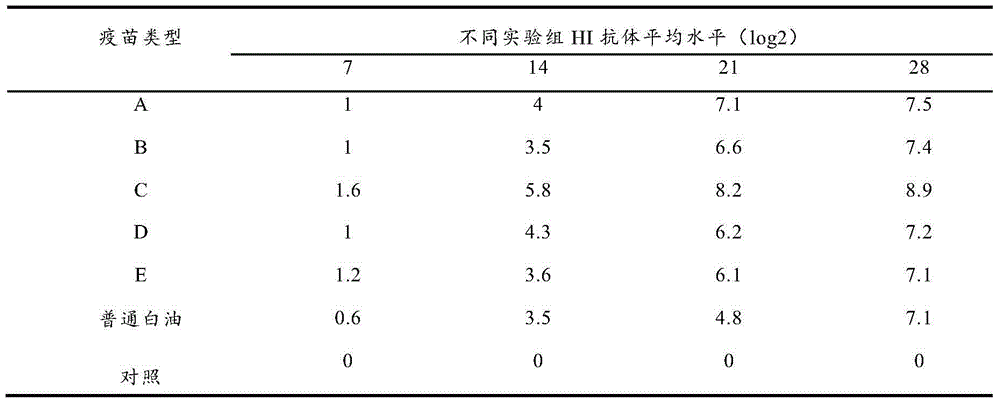
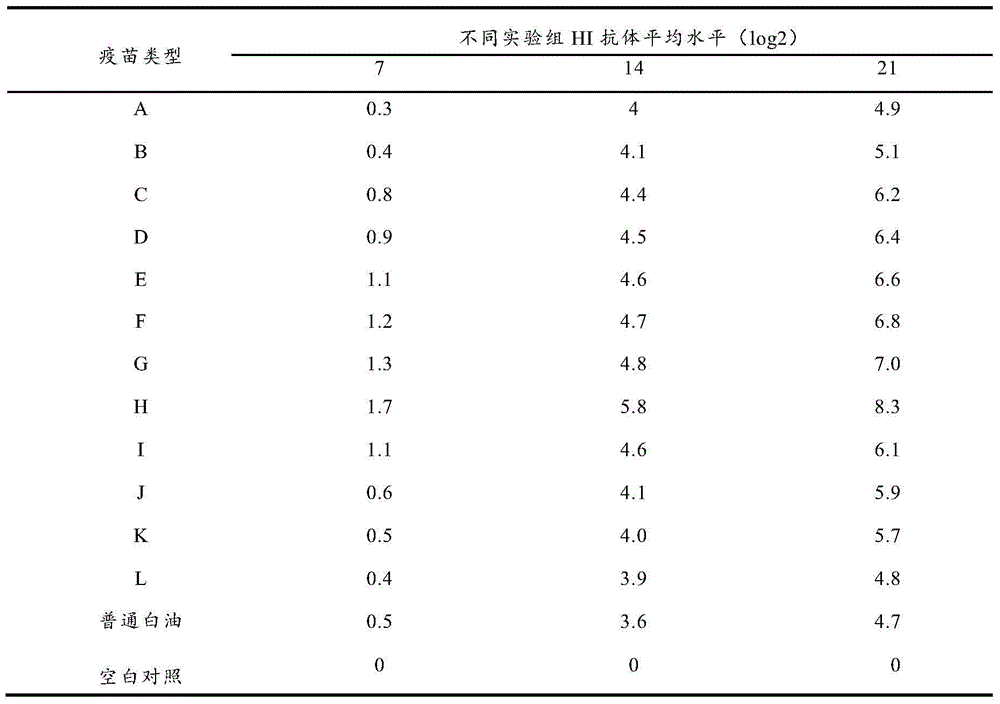
Image
Examples
Embodiment 1
[0025] Embodiment 1 aqueous phase solution preparation
[0026] 1, the preparation of levamisole mother liquor
[0027] Commercially available veterinary levamisole hydrochloride injection with a levamisole content of 50 mg / ml was used as the levamisole mother solution; it was filtered and sterilized with a 0.22 μm filter (purchased from Sartorius) before use.
[0028] 2. Preparation of aqueous solution
[0029] Get 4ml of 50mg / ml levamisole mother solution and mix with 96ml chicken Newcastle disease inactivated virus liquid, and adjust the pH value to reach neutrality, be the aqueous phase solution that contains levamisole in every milliliter of solution and be 2mg.
Embodiment 2
[0030] Embodiment 2 water phase solution preparation
[0031] 1, the preparation of levamisole mother liquor
[0032] Commercially available veterinary levamisole hydrochloride injection with a levamisole content of 50 mg / ml was used as the levamisole mother solution; it was filtered and sterilized with a 0.22 μm filter (purchased from Sartorius) before use.
[0033] 2. Preparation of aqueous solution
[0034] Get 10ml of 50mg / ml levamisole mother solution and 90ml chicken Newcastle disease inactivated virus solution and mix evenly, and adjust the pH value to reach neutrality, namely be the aqueous phase solution that contains levamisole in every milliliter of solution and be 5mg.
Embodiment 3
[0035] Embodiment 3 aqueous phase solution preparation
[0036] 1, the preparation of levamisole mother liquor
[0037] Commercially available veterinary levamisole hydrochloride injection with a levamisole content of 50 mg / ml was used as the levamisole mother solution; it was filtered and sterilized with a 0.22 μm filter (purchased from Sartorius) before use.
[0038] 2. Preparation of aqueous solution
[0039] Get 20ml of 50mg / ml levamisole mother solution and mix with 80ml chicken Newcastle disease inactivated virus liquid, and adjust the pH value to reach neutrality, be the aqueous phase solution that contains levamisole in every milliliter of solution and be 10mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com