Method for preparing conjugated linoleic acid
A technology of conjugated linoleic acid and linoleic acid ester, which is applied in the direction of fatty acid preparation/refining, fatty acid production, fatty acid esterification, etc., and can solve problems such as lack of conjugated linoleic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] One, the preparation of ethyl linoleate:
[0113] Raw material 1: Refined safflower oil (acid value below 0.1),
[0114]Preparation process: Take 50 parts of absolute ethanol, add 0.5 parts of catalyst KOH in oil weight, and dissolve it. Then add it to 100 parts of refined safflower oil, raise the temperature to 75°C, and stir for 4 hours. After the reaction, wash with water until neutral, remove the lower layer, take the upper liquid phase, dry it, and weigh it. Vacuum distillation (8-10pa) at about 170°C until no liquid drips out to obtain ethyl linoleate.
[0115] After testing, the prepared ethyl linoleate content was 77%.
[0116] Two, the preparation of conjugated linoleic acid ester:
Embodiment 1
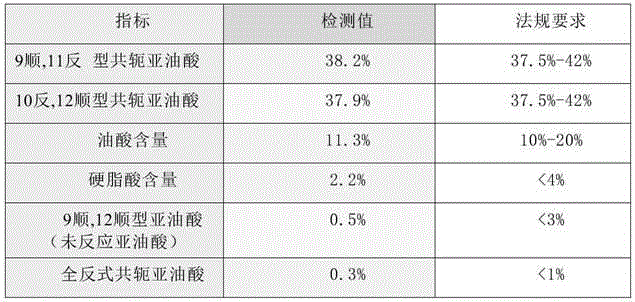
[0118] Weigh 100g of ethyl linoleate (raw material 1) into the reaction kettle, replace the air with nitrogen, raise the temperature to 100°C, add 4g of butyllithium, and keep stirring. After 3 hours of reaction, add 23g of glycerol triacetate, Turn on the vacuum and heat up to 140°C, cool to 60°C after 2.5 hours, add phosphoric acid aqueous solution to neutralize and wash with water, dry and molecularly distill off the remaining conjugated linoleic acid ethyl ester at 180°C to obtain conjugated linoleic acid triglyceride, Detect its fatty acid composition as follows.
[0119] Table 1 The percentage content of conjugated linoleic acid
[0120] index detection value Regulatory requirements 9cis,11trans conjugated linoleic acid 37.6% 37.5%~42% 10 trans,12 cis conjugated linoleic acid 37.9% 37.5%~42% Oleic acid content 11.3% 10%~20% Linoleic acid content 0.2% <3%
Embodiment 2
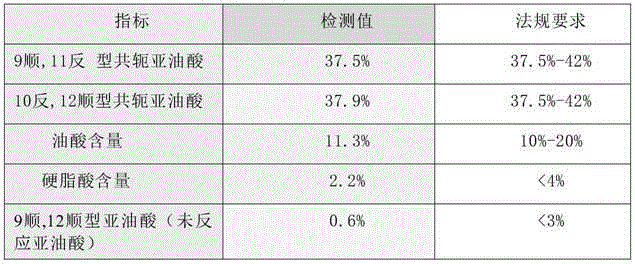
[0122] Weigh 100g of ethyl linoleate (raw material 1) into the reaction kettle, replace the air with nitrogen, raise the temperature to 125°C, add 5g of benzyllithium, and keep stirring. After 4 hours of reaction, add 23g of glycerol triacetate, Turn on the vacuum and heat up to 140°C, cool to 60°C after 2.5 hours, add phosphoric acid aqueous solution to neutralize and wash with water, dry and molecularly distill off the remaining conjugated linoleic acid ethyl ester at 180°C to obtain conjugated linoleic acid triglyceride, Detect its fatty acid composition as follows.
[0123] The percentage content of table 2 conjugated linoleic acid
[0124] index detection value Regulatory requirements 9cis,11trans conjugated linoleic acid 38.0% 37.5%~42% 10 trans,12 cis conjugated linoleic acid 37.9% 37.5%~42% Oleic acid content 11.1% 10%~20% Linoleic acid content 0.2% <3%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com